Question.
Why is the conversion of ethanol to ethanoic acid an oxidation reaction?
Why is the conversion of ethanol to ethanoic acid an oxidation reaction?
solution:
The reaction showing the conversion of ethanol to ethanoic acid can be shown as :
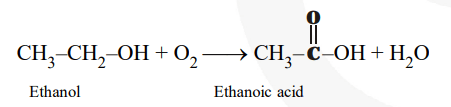
A molecule of ethanol contains one oxygen alone while that of ethanoic acid contains two oxygen atoms.
Since oxidation involves addition of oxygen therefore, conversion of ethanol to ethanoic acid is an oxidation reaction.
Alternatively, a molecule of ethanol contains six hydrogen atoms while that of ethanoic acid contains four hydrogen atoms since oxidation involves removal of hydrogen, therefore conversion of ethanol to ethanoic acid is an oxidation reaction.
The reaction showing the conversion of ethanol to ethanoic acid can be shown as :
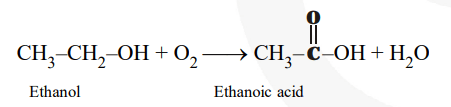
A molecule of ethanol contains one oxygen alone while that of ethanoic acid contains two oxygen atoms.
Since oxidation involves addition of oxygen therefore, conversion of ethanol to ethanoic acid is an oxidation reaction.
Alternatively, a molecule of ethanol contains six hydrogen atoms while that of ethanoic acid contains four hydrogen atoms since oxidation involves removal of hydrogen, therefore conversion of ethanol to ethanoic acid is an oxidation reaction.
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
