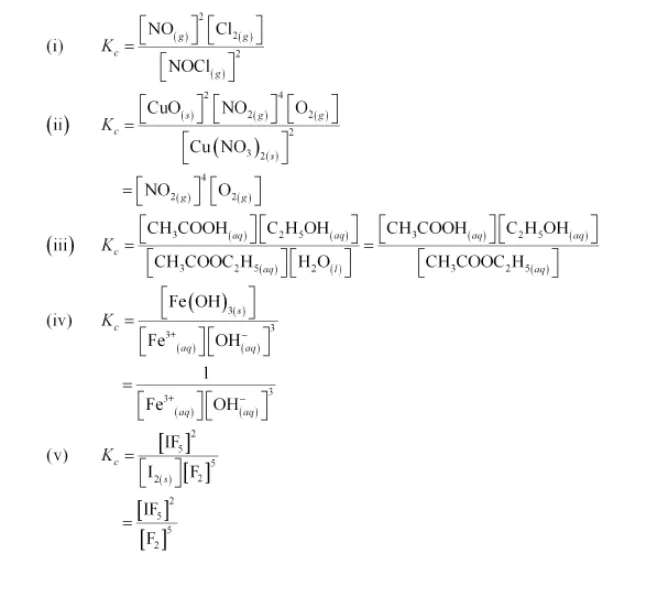
Write the expression for the equilibrium constant, Kc for each of the following
reactions:
(i) $2 \mathrm{NOCl}(\mathrm{g}) \longleftrightarrow 2 \mathrm{NO}(\mathrm{g})+\mathrm{Cl}_{2}(\mathrm{~g})$
(ii) $2 \mathrm{Cu}\left(\mathrm{NO}_{3}\right)_{2}(\mathrm{~s}) \longleftrightarrow 2 \mathrm{CuO}(\mathrm{s})+4 \mathrm{NO}_{2}(\mathrm{~g})+\mathrm{O}_{2}(\mathrm{~g})$
(iii) $\mathrm{CH}_{3} \mathrm{COOC}_{2} \mathrm{H}_{5}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{O}(\mathrm{l}) \longleftrightarrow \mathrm{CH}_{3} \mathrm{COOH}(\mathrm{aq})+\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}(\mathrm{aq})$
(iv) $\mathrm{Fe}^{3+}(\mathrm{aq})+3 \mathrm{OH}^{-}(\mathrm{aq}) \longleftrightarrow \mathrm{Fe}(\mathrm{OH})_{3}(\mathrm{~s})$
(v) $\mathrm{I}_{2}(\mathrm{~s})+5 \mathrm{~F}_{2} \longleftrightarrow 2 \mathrm{IF}_{5}$