JEE Main Previous Year Papers Questions of Chemistry With Solutions are available at eSaral.
Simulator
Previous Years AIEEE/JEE Mains Questions
Q. The products obtained on heating $\mathrm{LiNO}_{3}$ will be :-
(1) $\mathrm{LiNO}_{2}+\mathrm{O}_{2}$
(2) $\mathrm{Li}_{2} \mathrm{O}+\mathrm{NO}_{2}+\mathrm{O}_{2}$
(3) $\mathrm{Li}_{3} \mathrm{N}+\mathrm{O}_{2}$
(4) $\mathrm{Li}_{2} \mathrm{O}+\mathrm{NO}+\mathrm{O}_{2}$
[AIEEE-2011]
Ans. (2)
Fact based.
Q. What is the best description of the change that occurs when $\mathrm{Na}_{2} \mathrm{O}(\mathrm{s})$ is dissolved in water ?
(1) Oxidation number of sodium decreases
(2) Oxide ion accepts sharing in a pair of electrons
(3) Oxide ion donates a pair of electrons
(4) Oxidation number of oxygen increases
Ans. (3)
$\mathrm{O}^{-2}+\mathrm{H}^{\ominus} \rightarrow \mathrm{O}-\mathrm{H}\left\{\mathrm{O}^{-2} \rightarrow \mathrm{H}^{\ominus}\right\}$
Q. Which of the following on thermal-decomposition yields a basic as well as an acidic oxide ?
(1) $\mathrm{NH}_{4} \mathrm{NO}_{3}$
(2) NaNO $_{3}$
(3) $\mathrm{KClO}_{3}$
(4) CaCO $_{3}$
[AIEEE-2012]
Ans. (4)
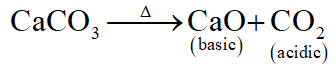
Q. Fire extinguishers contain $\mathrm{H}_{2} \mathrm{SO}_{4}$ and which one of the following :-
(1) $\mathrm{CaCO}_{3}$
(2) $\mathrm{NaHCO}_{3}$ and $\mathrm{Na}_{2} \mathrm{CO}_{3}$
(3) $\mathrm{Na}_{2} \mathrm{CO}_{3}$
(4) $\mathrm{NaHCO}_{3}$
[JEE MAIN-2012, Online]
Ans. (4)
Solution not required
Q. Based on lattice energy and other considerations, which one of the following alkali metal chloride is expected to have the highest melting point ?
(1) RbCl
(2) LiCl
(3) KCl
(4) NaCl
[JEE MAIN-2012, Online]
Ans. (4)
NaCl having highest lattice energy have highest melting point among these.
Q. Which one of the following will react most vigorously with water ?
(1) Li (2) K (3) Rb (4) Na
[JEE MAIN-2012, Online]
Ans. (3)
Down the group reactivity of s-block metals increases.
Q. A metal M on heating in nitrogen gas gives Y. Y on treatment with $\mathrm{H}_{2} \mathrm{O}$ gives a colourless gas which when passed through $\mathrm{CuSO}_{4}$ solution gives a blue colour, Y is :-
(1) $\mathrm{NH}_{3}$
(2) MgO
(3) $\mathrm{Mg}_{3} \mathrm{N}_{2}$
(4) $\mathrm{Mg}\left(\mathrm{NO}_{3}\right)_{2}$
[JEE MAIN-2012, Online]
Ans. (3)
$\mathrm{M}+\mathrm{N}_{2} \rightarrow$ metal nitride (y) so according to the given options answer is (3)
Q. The correct statement for the molecule, $\mathrm{CSI}_{3}$, is :
(1) it contains $\mathrm{Cs}^{3+}$ and I– ions
(2) it contains $\mathrm{Cs}^{+}$, I– and lattice I2 molecule
(3) it is a covalent molecule
(4) it contains $\mathrm{Cs}^{+}$ and ions.
[JEE(Main)-2014]
Ans. (4)
$\mathrm{CsI}_{3}$ is ionic compound which have $\mathrm{Cs}^{\oplus}$ and $\mathrm{I}_{3}^{\oplus}$ ion.
Q. Which of the following statements about $\mathrm{Na}_{2} \mathrm{O}_{2}$ is not correct ?
(1) $\mathrm{Na}_{2} \mathrm{O}_{2}$ oxidises $\mathrm{Cr}^{3+}$ to $\mathrm{CrO}_{4}^{2-}$ in acid medium
(2) It is diamagnetic in nature
(3) It is the super oxide of sodium
(4) It is a derivative of $\mathrm{H}_{2} \mathrm{O}_{2}$
[JEE MAIN-2014, Online]
Ans. (3)
$\mathrm{Na}_{2} \mathrm{O}_{2}$ is sodium peroxide not the superoxide.
Q. Amongst LiCl, RbCl, $\mathrm{BeCl}_{2}$ and $\mathrm{MgCl}_{2}$ the compounds with the greatest and the least ionic character, respectively are :
(1) $\mathrm{RbCl}$ and $\mathrm{MgCl}_{2}$
(2) LiCl and RbCl
(3) $\mathrm{MgCl}_{2}$ and $\mathrm{BeCl}_{2}$
(4) $\mathrm{RbCl}$ and $\mathrm{BeCl}_{2}$
[JEE MAIN-2014, Online]
Ans. (4)
Due to smallest cationic size $\mathrm{BeCl}_{2}$ is least ionic while due to biggest ionic size of $\mathrm{Rb}^{\oplus}$, RbCl has greatest ionic character.
Q. The correct order of thermal stability of hydroxides is :
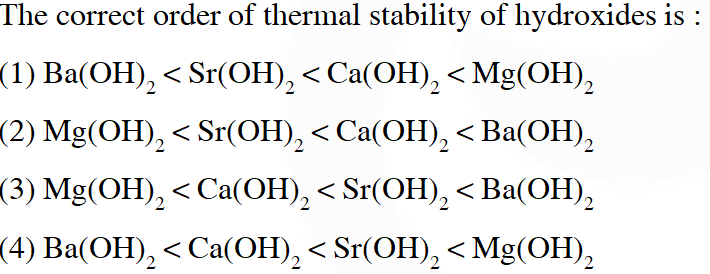 [JEE(Main)Online-2015]
[JEE(Main)Online-2015]
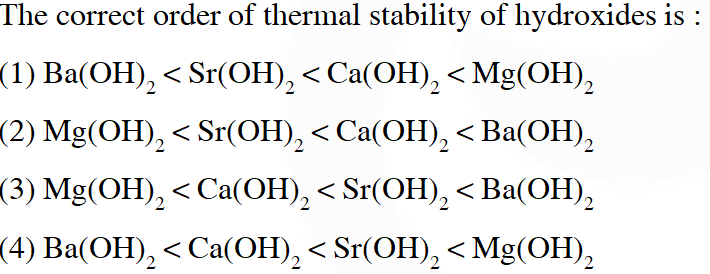 [JEE(Main)Online-2015]
[JEE(Main)Online-2015]
Ans. (3)
For the ionic compound having polyatomic anion, thermal stability increases down the group.
Q. Which of the alkaline earth metal halide given below is essentially covalent in nature :-
(1) $\mathrm{SrCl}_{2}$
(2) $\mathrm{CaCl}_{2}$
(3) $\mathrm{BeCl}_{2}$
(4) $\mathrm{MgCl}_{2}$
[JEE(Main)Online-2015]
Ans. (3)
Fact based.
Comments
Vidya
Feb. 11, 2021, 7:18 a.m.
If you please provide more questions it would be more helpful to us.....
Ankana Dutta
Oct. 7, 2020, 1:28 p.m.
Please provide latest year questions..Thanks..for your help..😊😊😊
kfkorefmeokfke
Aug. 27, 2020, 7:11 a.m.
rr4rrg fkdfkf gfrelkf fmkfee f ekfq kfqfm rmfr rkrkk4 jkfmkefq frfrmkfrr f rk rqtfr girk kmirmtr frfqrkf fkermfkqf qfmmkqr fmoqm3f 4rtmkt4 rt 4tm4t 4t4o kqmrtot4 rt4kmroo4ro4r4 r4krk4rk4r4 4kmro4o4 rm4ro4o4 4ro4or4oo4 ro4o prl43rp4lr roikgiigkjb bifrmfgbkif mi
pallavi
Aug. 16, 2020, 9:01 p.m.
Thanks for the questions ...
If possible please provide more questions..
Rohan
June 22, 2020, 1:15 a.m.
If every year questions were available then it would be more helpful!!!anyways thank you☺☺
