Acids Bases and Salts Class 10 Notes
Class 10
Introduction, Definition, History
Acids, bases, and salts ( class 10 notes ) are chemical compounds that are crucial to our understanding of chemistry. An acid is a substance that can donate hydrogen ions, while a base is a substance that can accept hydrogen ions. A salt is a compound that forms when an acid reacts with a base.
The study of acids, bases, and salts is important because these compounds are involved in many chemical reactions that take place in our daily lives. For example, acids are used in the production of fertilizers, cleaning agents, and medicines. Bases are used in the production of soaps, detergents, and antacids. Salts have a wide range of uses, including in the production of food, medicines, and fertilizers.
The history of acids, bases, and salts dates back to ancient times, where the use of vinegar and lime juice in food preservation was common. The first systematic study of acids and bases was carried out by the French chemist Antoine Lavoisier in the late 18th century. Later, in the 19th century, the Swedish chemist Svante Arrhenius proposed the theory of electrolytes, which explained the behavior of acids and bases in solution.
Types of Indicators- Acids Bases and Salts Class 10 Notes
There are several types of indicators that scientists use to determine whether a substance is an acid or a base.
One type of indicator is a universal indicator, which is a mixture of several indicators that can change color over a wide pH range. It can be used to determine the approximate pH of a solution.
Another type of indicator is litmus paper, which comes in two colors, blue and red. Blue litmus paper turns red in the presence of an acid, while red litmus paper turns blue in the presence of a base.
Turmeric: Turmeric is another natural indicator. Turmeric is yellow in colour. Turmeric solution or paper turns reddish brown with base. Turmeric does not change colour with acid.
Red Cabbage: The juice of red cabbage is originally purple in colour. Juice of red cabbage turns reddish with acid and turns greenish with base.
Olfactory Indicators- Acids Bases and Salts Class 10 Notes
Olfactory Indicators are substances that change their smell when mixed with an acid or a base. For example, onion and vanilla are two commonly used olfactory indicators.
When the paste or juice of an onion is mixed with a base, it loses its smell. However, it does not change its smell when mixed with an acid. On the other hand, the smell of vanilla disappears when mixed with a base, but its smell remains unchanged when mixed with an acid.
Olfactory Indicators are particularly useful in laboratory experiments involving visually impaired students, as they allow them to detect changes in acidity or basicity through changes in smell.
Synthetic Indicator- Acids Bases and Salts Class 10 Notes
Phenolphthalein is another commonly used indicator that changes color from colorless to pink in the presence of a base.
Methyl orange is an indicator that changes color from red to yellow in the presence of an acid.
Bromothymol blue is an indicator that changes color from yellow to blue in the presence of a base.
| Indicator | Original Colour | Acid | Base |
| Red litmus | Red | No Change | Blue |
| Blue litmus | Blue | Red | No change |
| Turmeric | Yellow | No Change | Reddish brown |
| Red cabbage juice | Purple | Reddish | Greenish yellow |
| Phenolphthalein | Colourless | Colourless | Pink |
| Methyl Orange | Orange | Red | Yellow |
| Onion | n/a | No change | Smell vanishes |
| Vanilla | n/a | No change | Smell vanishes |
Acids - Acids Bases and Salts Class 10 Notes
Acids are chemical compounds that can donate hydrogen ions (H+) to a solution. They are characterized by a sour taste and a pH value less than 7. Acids can also react with metals to produce hydrogen gas and with bases to form salts and water.
Some common examples of acids include hydrochloric acid, sulfuric acid, and acetic acid (found in vinegar). Acids have many practical uses, such as in the production of fertilizers, cleaning agents, and medicines.
Overall, understanding the properties and behaviors of acids is essential in many areas of chemistry and other fields, such as agriculture, healthcare, and manufacturing.
Types of Acids -Acids Bases and Salts Class 10 Notes
Natural acids:
Acids which are obtained from natural sources are called Natural Acids or Organic Acids.
Examples:
Methanoic acid (HCOOH)
Acetic acid (CH3COOH)
Oxalic acid (C2H2O4) etc
| Organic Acids and their Sources | |
| Acids | Sources |
| Acetic acid | Vinegar |
| Ascorbic acid | Guava, amla |
| Citric acid | Lemon, orange and other citrus fruits |
| Lactic acid | Sour milk, curd |
| Methanoic acid | Ant sting, nettle sting |
| Oxalic acid | Tomato |
| Tartaric acid | Tamarind |
(ii) Mineral Acids:
Mineral Acids are a type of acid that are prepared from minerals, and are also known as inorganic acids, man-made acids, or synthetic acids. Examples of Mineral Acids include Hydrochloric acid (HCl), Sulphuric acid (H2SO4), Nitric acid (HNO3), Carbonic acid (H2CO3), Phosphoric acid (H3PO4), among others.
These acids have a wide range of industrial and scientific applications. For example, Sulphuric acid is commonly used in the production of fertilizers, while Nitric acid is used in the production of explosives. Hydrochloric acid is also widely used in the production of plastics, pharmaceuticals, and other chemicals.
Chemical Properties of acids
When acids react with metals, they produce hydrogen gas along with a salt specific to the metal. This reaction can be represented as: Metal + Acid → Salt + Hydrogen
For example, when zinc metal is reacted with hydrochloric acid, hydrogen gas and zinc chloride are produced. This chemical reaction can be represented by the equation: Zn + 2HCl → ZnCl2 + H2.
When sulphuric acid reacts with sodium metal, hydrogen gas and sodium sulphate are formed. This chemical reaction can be represented by the equation: 2Na + H2SO4 → Na2SO4 + H2
To test for the presence of hydrogen gas produced by the reaction of acid with metal, a lighted candle can be brought near the gas. If the gas burns with a pop sound, it confirms the evolution of hydrogen gas. This is a characteristic test for hydrogen gas.
In addition to reacting with metals,
When sulphuric acid reacts with sodium metal, hydrogen gas and sodium sulphate are formed. This chemical reaction can be represented by the equation: 2Na + H2SO4 → Na2SO4 + H2
To test for the presence of hydrogen gas produced by the reaction of acid with metal, a lighted candle can be brought near the gas. If the gas burns with a pop sound, it confirms the evolution of hydrogen gas. This is a characteristic test for hydrogen gas.
to produce carbon dioxide gas and a salt specific to the metal. This reaction can be represented as: Metal carbonate + Acid → Salt + Carbon dioxide + Water
For example, when hydrochloric acid reacts with sodium carbonate, carbon dioxide gas, sodium chloride, and water are produced. This reaction can be represented by the equation: Na2CO3 + 2HCl → 2NaCl + CO2 + H2O
In addition to reacting with metals, acids can also react with metal carbonates to produce carbon dioxide gas and a salt specific to the metal. This reaction can be represented as: Metal carbonate + Acid → Salt + Carbon dioxide + Water
For example, when hydrochloric acid reacts with sodium carbonate, carbon dioxide gas, sodium chloride, and water are produced. This reaction can be represented by the equation: Na2CO3 + 2HCl → 2NaCl + CO2 + H2O
When sulphuric acid reacts with calcium carbonate, it produces calcium sulphate, carbon dioxide gas, and water. This chemical reaction can be represented by the equation: CaCO3 + H2SO4 → CaSO4 + CO2 + H2O
Similarly, when nitric acid reacts with sodium carbonate, it produces sodium nitrate, water, and carbon dioxide gas. This chemical reaction can be represented by the equation: Na2CO3 + 2HNO3 → 2NaNO3 + CO2 + H2O
Acids can also react with metal hydrogen carbonates (bicarbonates) to produce carbon dioxide gas, a salt specific to the metal, and water. This reaction can be represented as: Acid + Metal hydrogen carbonate → Salt + Carbon dioxide + Water
For example, when sulphuric acid reacts with sodium bicarbonate, it produces sodium sulphate, carbon dioxide gas, and water. This chemical reaction can be represented by the equation: NaHCO3 + H2SO4 → Na2SO4 + CO2 + H2O
Understanding these reactions is important in chemistry and has practical applications in various industries such as agriculture, food, and medicine.
The characteristic test for carbon dioxide gas is turning lime water milky when passed through it. This happens because of the formation of a white precipitate of calcium carbonate due to the reaction of the acid with metal carbonate or metal hydrogen carbonate. Excess carbon dioxide passed through lime water causes the milky color to disappear because of the formation of calcium hydrogen carbonate, which is soluble in water.
Common in acids: All acids contain hydrogen and give hydrogen gas when they react with metal. When an acid is dissolved in water, it dissociates hydrogen, which is the common property in all acids. The dissociation of hydrogen ion in aqueous solution makes an acid exhibit acidic behavior. Examples: Hydrochloric acid (HCl) dissociates into hydrogen ion (H+) and chloride ion (Cl–) when dissolved in water. Acetic acid (CH3COOH) dissociates into acetate ion (CH3COO–) and hydrogen ion (H+).
Chemical equation for the reaction of Hydrochloric acid with Sodium Carbonate:
2HCl + Na2CO3 → 2NaCl + CO2 + H2O
Strong Acids and Weak acids
Strong acids are those which completely ionize in water and produce (H+) ions. Examples of strong acids are Hydrochloric acid (HCl), Sulphuric acid (H2SO4), and Nitric acid (HNO3).
On the other hand, weak acids are those which only partially ionize in water and thus produce a small amount of hydrogen ions (H+). An example of a weak acid is Acetic acid (CH3COOH) and Carbonic acid (H2CO3).
When a concentrated solution of acid is diluted by mixing water, then the concentration of Hydrogen ions (H+) or hydronium ion (H3O–) per unit volume decreases
Bases
Bases give hydroxide ions (OH–) in aqueous solution, have a bitter taste, a soapy touch, and turn red litmus blue. Sodium hydroxide (NaOH), calcium hydroxide (Ca(OH)2), and potassium hydroxide (KOH) are examples of bases.
Bases can be divided into two types, water-soluble and water-insoluble. The hydroxides of alkali and alkaline earth metals are soluble in water and are also known as alkalis. Alkalis are considered strong bases.
Bases react with metals to produce salt and hydrogen gas
The chemical equation for this reaction is: Base + Metal → Salt + Hydrogen
Chemical properties of bases
(i) Reaction of base with metals: For example, when sodium hydroxide reacts with zinc metal, it produces hydrogen gas and sodium zincate. Sodium hydroxide also reacts with aluminum metal to produce sodium aluminate and hydrogen gas.
Examples: Sodium hydroxide gives hydrogen gas and sodium zincate when reacts with zinc metal.
2NaOH + Zn → Na2ZnO2 + H2
Sodium aluminate and hydrogen gas are formed when sodium hydroxide reacts with aluminium metal.
2NaOH + Zn -> Na2ZnO2 + H2
(ii) Reaction of bases with oxides of Non metals :
Non-metal oxides exhibit acidic nature. For instance, carbon dioxide, a non-metal oxide, produces carbonic acid when it dissolves in water.
Therefore, a base reacts with a non-metal oxide to neutralize each other, resulting in the formation of respective salt and water.
Base + Non-metal oxide → Salt + Water
When carbon dioxide reacts with sodium hydroxide, it produces sodium carbonate and water.
CO2 + 2NaOH → Na2CO3 + H2O
Calcium hydroxide gives calcium carbonate and water when it reacts with carbon dioxide.
Ca(OH)2 + CO2 → CaCO3 + H2O
(iii) Neutralization Reaction:
Active voice with equations:
When an acid reacts with a base, they neutralize each other, resulting in the formation of respective salt and water.
Acid + Base → Salt + Water
This type of reaction is known as a neutralization reaction because both acid and base neutralize each other.
Examples: Hydrochloric acid, a strong acid, reacts with sodium hydroxide, a strong base, to produce sodium chloride and water.
HCl + NaOH → NaCl + H2O
(iv) Reaction of Acid with Metal Oxides:
Metal oxides exhibit basic nature. Therefore, when an acid reacts with a metal oxide, they neutralize each other and form respective salt and water.
Acid + Metal Oxide → Salt + Water
Examples: Calcium oxide, a metallic oxide, is basic in nature due to the presence of a metal. When hydrochloric acid reacts with calcium oxide, a neutralization reaction occurs, resulting in the formation of calcium chloride and water.
HCl + CaO → CaCl2 + H2O
Common in all bases
A compound exhibits basic behavior due to the dissociation of hydroxide ions in water, which is caused by a base.
When dissolved in water, sodium hydroxide dissociates into sodium ions and hydroxide ions.
NaOH → Na+ + OH-
Neutralisaton Reaction
When an acid reacts with a base, the hydrogen ion (H+) of the acid combines with the hydroxide ion (OH-) of the base to form water (H2O). This combination of ions results in the neutralization of both the acid and the base.
H+ (acid) + OH- (base) → H2O (water)
Dilution of Acid and Base:
The concentration of acid or base is determined by the concentration of hydrogen ion in an acid and hydroxide ion in a base, per unit volume.
When acid is mixed with water, the concentration of hydrogen ion per unit volume decreases. Similarly, the addition of base to water results in a decrease in the concentration of hydroxide ion per unit volume. This process is known as dilution, and the resulting acid or base is referred to as diluted.
Dilution of acid or base is an exothermic process. Thus, it is important to add acid or base to water rather than water to the acid or base. If water is added to a concentrated acid or base, it generates a lot of heat, which may cause the acid or base to splash out and cause severe damage as concentrated acid and base are highly corrosive.
Note: The original text was already in the active voice, so no major changes were needed, only some rephrasing for clarity.
Strength of Acid and Base
Acids that completely dissociate the hydrogen ion are referred to as strong acids. Likewise, bases that completely dissociate the hydroxide ion are known as strong bases.
Mineral acids, such as hydrochloric acid, sulfuric acid, and nitric acid, completely dissociate the hydrogen ion, making them strong acids. In contrast, inorganic acids do not fully dissociate the hydrogen ions, making them weak acids.
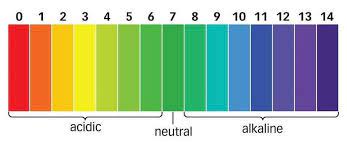
For water or neutral solutions : pH = 7
For acidic solutions : pH < 7
For basic solution : pH > 7
Universal Indicator: Litmus paper, phenolphthalein, methyl orange, and other indicators can only determine the acidic or basic nature of a solution. However, these indicators cannot provide information on the strength of the acid or base. Therefore, to determine both the strength and acidic or basic nature of a given solution, a universal indicator is used.
A universal indicator can display a range of colors corresponding to pH values from 1 to 14 for a given solution. Universal indicators are available in both strip and solution form. The combination of several indicators such as water, propanol, phenolphthalein, sodium salt, sodium hydroxide, methyl red, bromothymol blue monosodium salt, and thymol blue monosodium salt make up a universal indicator. A color matching chart is included with the universal indicator to display the various colors corresponding to different pH values.
) pH in our digestive system: Dilute HCl (Hydrochloric acid) helps in digestion of food (proteins) in our stomach. Excess acid in stomach causes acidity (indigestion). Antacids like magnesium hydroxide [Mg(OH)2] also known as milk of magnesia and sodium hydrogen carbonate (baking soda) are used to neutralize excess acid.
(ii) Tooth decay caused by acids: The bacteria present in our mouth converts the sugar into acids. When the pH of acid formed in the mouth falls below 5.5, tooth-decaying starts. The excess acid has to be removed by cleaning the teeth with a good quality toothpaste because these kinds of toothpaste are alkaline in nature.
(iii) Soil of pH and plant growth: Most of the plants have a healthy growth when the soil has a specific pH (close to 7) range which should be neither alkaline nor highly acidic. Therefore,
- Compound ‘X’ is Sodium hydroxide (NaOH).
- Compound ‘A’ is Zinc sulphate (ZnSO4).
- Compound ‘B’ is Sodium chloride (NaCl).
- Compound ‘C’ is Sodium acetate (CH3COONa)
Salts
Salts are ionic compounds produced by the neutralization reaction between an acid and a base. They are electrically neutral. The most common salt is sodium chloride, also known as table salt or common salt, which is used to enhance the taste of food.
The characteristics of salts are:
- Most salts are crystalline solids.
- Salts can be transparent or opaque.
- Most salts are soluble in water.
- Salt solutions conduct electricity in their molten state.
- Salts can have different tastes such as salty, sour, sweet, bitter, and umami (savory).
- Neutral salts are odourless.
- Salts can be colourless or coloured.
Salts having common acidic or basic radicals are said to belong to the same family. For example, sodium chloride (NaCl) and calcium chloride (CaCl2) belong to the chloride family, calcium chloride (CaCl2) and calcium sulfate (CaSO4) belong to the calcium family, and zinc chloride (ZnCl2) and zinc sulfate (ZnSO4) belong to the zinc family.
Neutral, Acidic and Basic Salts
(i) Neutral Salt :When a strong acid reacts with a strong base, the resulting salts are neutral in nature and have a pH value of 7. Example: Sodium chloride, sodium sulfate, potassium chloride, etc.
For instance, sodium chloride (NaCl) is formed when hydrochloric acid (HCl), a strong acid, reacts with sodium hydroxide (NaOH), a strong base: HCl + NaOH → NaCl + H2O
(ii) Acidic Salts: When a strong acid reacts with a weak base, it forms an acidic salt with a pH value less than 7. Ammonium chloride is an example of such a salt, which is formed by the reaction between hydrochloric acid (HCl) and ammonium hydroxide (NH4OH):
HCl + NH4OH → NH4Cl + H2O
The resulting salt, ammonium chloride (NH4Cl), is acidic in nature and has a pH value less than 7.
(iii) Basic Salts: Basic Salts are salts formed as a result of the reaction between a strong base and a weak acid. For instance, sodium carbonate and sodium acetate.
The reaction between sodium hydroxide, a strong base, and carbonic acid, a weak acid, produces sodium carbonate:
NaOH + H2CO3 → Na2CO3 + H2O
Some Important Chemical Compounds
1. Common Salt (Sodium Chloride): Sodium chloride (NaCl) is also known as Common or Table Salt. It is formed after the reaction between sodium hydroxide and hydrochloric acid. It is a neutral salt. The pH value of sodium chloride is about 7. Sodium chloride is used to enhance the taste of food. Sodium chloride is used in the manufacturing of many chemicals
The important chemical obtained from sodium chloride is sodium hydroxide (NaOH), which is a strong base and commonly known as caustic soda.
To obtain sodium hydroxide, a solution of sodium chloride (brine) undergoes electrolytic decomposition. In this process, brine is decomposed into sodium hydroxide, chlorine at the anode, and hydrogen gas at the cathode. This process is commonly referred to as the Chlor-Alkali process.Acids Bases and Salts Class 10 Notes.
The electrolytic decomposition of brine can be represented by the following equation:
2NaCl + 2H2O → 2NaOH + Cl2 + H2
Use of products after the electrolysis of brine:
- Hydrogen gas is used as fuel, margarine, in making of ammonia for fertilizer, etc.
- Chlorine gas is used in water treatment, manufacturing of PVC, disinfectants, CFC, pesticides. It is also used in the manufacturing of bleaching powder and hydrochloric acid.
- Sodium hydroxide is used for degreasing of metals, manufacturing of paper, soap, detergents, artificial fibres, bleach, etc.
2. Baking Soda (NaHCO3): Baking soda, also known as sodium hydrogen carbonate (NaHCO3) or sodium bicarbonate, is an important product obtained using the byproducts of the Chlor-Alkali process. It has various other names such as bread soda, cooking soda, bicarbonate of soda, sodium bicarb, bicarb of soda, etc. Here are the active voice statements with equations about baking soda:
- Baking soda is prepared by reacting brine with carbon dioxide and ammonia, a process known as the Solvay process. Calcium carbonate is used as the source of CO2, and the resultant calcium oxide is used to recover ammonia from ammonium chloride. This process can be represented by the following equation:
2NH3 + CO2 + H2O + NaCl → 2NaHCO3 + NH4Cl
-
Sodium hydrogen carbonate is a white crystalline solid that appears as a fine powder. It is amphoteric in nature and sparingly soluble in water.
-
When baking soda is heated, it decomposes into sodium carbonate, carbon dioxide, and water, as shown by the following equation:
2NaHCO3 + heat → Na2CO3 + CO2 + H2O
- Sodium carbonate formed after the thermal decomposition of sodium hydrogen carbonate decomposes into sodium oxide and carbon dioxide upon further heating. This reaction is known as a dehydration reaction and can be represented as follows:
Na2CO3 → Na2O + CO2
- Baking soda has various uses such as in making baking powder, which is used in cooking as it produces carbon dioxide that makes the batter soft and spongy. It is also used as an antacid, in toothpaste to make teeth white and plaque-free, and in cleaning silver ornaments. Additionally, it can be used as a fire extinguisher since sodium hydrogen carbonate gives carbon dioxide and sodium oxide on strong heating.
-
Obtain anhydrous sodium carbonate by thermal decomposition of sodium hydrogen carbonate using the Solvay process.
-
Rehydrate anhydrous sodium carbonate to obtain washing soda, which has ten water molecules attached to it.
Na2CO3 + 10H2O → Na2CO3.10H2O
Sodium carbonate is soluble in water, and washing soda is no exception. The addition of water allows for the water molecules to be attached to the anhydrous sodium carbonate, resulting in the formation of washing soda.
Here are some uses of washing soda:
-
It is used as a cleaning agent for clothes, particularly in rural areas.
-
It is a key ingredient in the production of detergent powders and cakes.
-
It is used to remove the permanent hardness of water.
-
The glass and paper industries use it in their production processes.
The water of Crystallization: Hydrated salts contain water molecules and are called Hydrated Salts. The water molecule present in a salt is referred to as the Water of Crystallization. Here are some examples:
- Copper Sulphate Pentahydrate (CuSO4.5H2O): The blue color of copper sulphate is due to the presence of 5 water molecules. When copper sulphate pentahydrate is heated, it loses these water molecules and turns into a gray-white color, which is known as anhydrous copper sulphate.
CuSO4.5H2O (Copper Sulphate Pentahydrate) → CuSO4 (Anhydrous Copper Sulphate) + 5H2O
- After adding water to anhydrous copper sulphate, it becomes blue again, indicating the return of water of crystallization.
The presence of water of crystallization affects the physical properties of a salt. For example, hydrated salts tend to have higher melting and boiling points than anhydrous salts.
Acids: Substances which turn blue litmus solution red are called acids. Acids are sour in taste.
Bases: Substances which change red litmus solution blue are called bases. They are bitter in taste.
Mineral Acids: Acids which are obtained from minerals like sulphates, nitrates, chlorides etc. are called mineral acids, example, H2SO4 (Sulphuric acid), HNO3 (Nitric acid) and HCl (Hydrochloric acid).
Organic Acids: Acids which are obtained from plants and animals are called organic acids. Example citric acid, ascorbic acid, tartaric acid, lactic acid, acetic acid.
Hydronium Ions: They are formed by the reaction of H+ (from acid) and H2O. It is because H+ is unstable.
Universal Indicator: A universal indicator is a mixture of indicators which shows a gradual but well-marked series of colour changes over a very wide range of change in concentration of H+ ions.
Strong Acids: Acids which dissociate into ions completely are called strong acids. Example, H2SO4, HCl.
Weak Acids: Acids which do not dissociate into ions completely are called weak acids. Example, citric acid, acetic acid.
Chemical Properties of Acids:
- Acids react with active metals to give salt and hydrogen gas.
- Acids react with metal carbonates and metal hydrogen carbonates to give salt, water and carbon dioxide.
- Acids react with bases to give salt and water. This reaction is called a neutralization reaction.
- Acids react with metal oxides to give salt and water.
Chemical Properties of Bases:
- Reaction with metals: Certain metals such as zinc, aluminium and tin react with alkali solutions on heating and hydrogen gas is evolved.
- Reaction with acids: Bases react with acids to form salt and water.
Indicators: Indicators are substances which indicate the acidic or basic nature of the solution by their colour change.
pH Scale: A scale for measuring hydrogen ion concentration in a solution.
The pH of a solution is defined as the negative logarithm of hydrogen ion concentration in moles per litre.
pH = -log [H+]
pH = -log [H3O+]
where [H+] or [H3O+] represents concentrations of hydrogen ions in a solution.
- The pH of a neutral solution is 7.
- The pH of an acidic solution is < 7.
- The pH of a basic solution is > 7.
Some Important Compounds and their Uses: Equations of Acids, Bases and Salts:
- Acid + Metal → Salt + Hydrogen gas
H2SO4 + Zn → ZnSO4 + H2 - Base + Metal → Salt + Hydrogen gas
2NaOH + Zn → Na2ZnO2 (Sodium zincate) + H2 - Base + Acid → Salt + Water
NaOH (aq) + HCl (aq) → NaCl (aq) + H2O (l) - Acids give hydronium ions in water
HCl + H2O → H3O+ + Cl– - Bases generate OH- ions in water
NaOH (aq) + H2O → Na+ (aq) + O– (aq)
Download the eSaral App for complete Class 10 Video lectures, Study material, revision and much more.
