Applications of Colloids
Colloids play an important role in our daily life and industry. Some of the important applications of colloids are listed below.
Adsorption Indicators
Indicators like fluoresce in function by adsorption of ions on to sol particles When silver nitrate solution is run into a solution of sodium chloride containing a fluoresce in a white precipitate of AgCl is first formed. At the end point the white precipitate turns sharply pink.
Tanning
Animal leather (skin) is soft due to the presence of very fine globules oils and fats in the' pores of skin. These globules are of colloidal in nature. When this soft leather (skin) is placed in salt water, these globules of oils and fats coagulate and settle down in water. Now when the animal hides tare dried, they become hand. This process of making soft leather to hard leather by dipping it in salt water is called tanning usually chromium salts are used for tanning.
Clearing action of soaps and detergents
Dust and dirt particles on clothes are colloidal in nature soaps being sodium salts of higher fatty acids coagulates them which become suspension particles called micelle and roll out due to greater volume and greater mass.
Industrial products
Paints, inks, synthetic plastics, rubber, graphite lubricants, cement, etc are all colloidal solutions.
Colloidal solution of graphite in water is called “Aqua dag” while that in Oil is called oil dag.
Adsorption & Catalysis
Adsorption
The phenomenon of increase in concentration at the surface due to molecular surface force is known as adsorption.
Adsorbent
The solid substance on the surface of which adsorption takes place is called adsorbent. Examples of absorbents are activated charcoal, Pt, Pd, Ni etc.
Adsorbate
The substances, gases or-liquids which are adsorbed on the surface of adsorbent are called adsorbent.
Sorption
lt may be defined as the process in which both adsorption and absorption take place simultaneously.
Absorption
When a substance is uniformly distributed throughout the body of a solid of liquid, the phenomenon is called absorption.
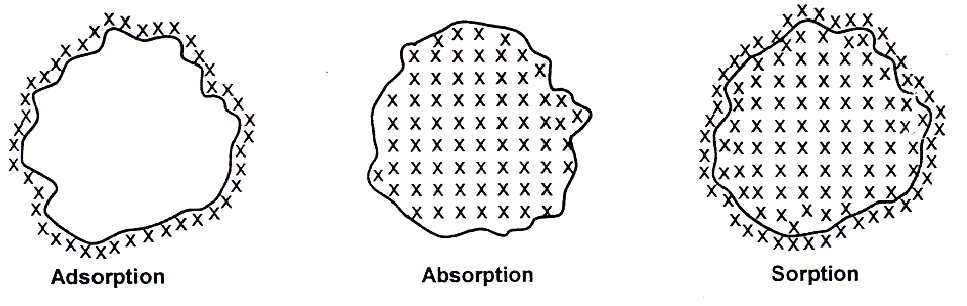
Mechanism of Adsorption
Adsorption is due to the fact that the surface particles of the adsorbent are in different state than the particles inside the bulk. Inside the adsorbent all the forces acting between the particles are mutually balanced but on the surface particles are not surrounded by atoms or molecules of their kind on all sides and hence they possess unbalanced or residual attractive forces.
Types of adsorption
Depending upon the nature of forces between molecules of adsorbate and adsorbent, adsorption is of two types.
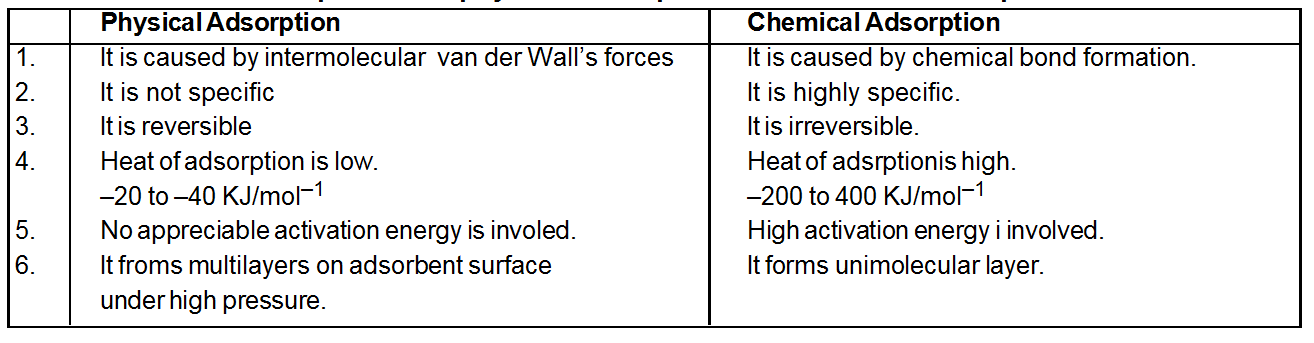
Comparison of physical adsorption and Chemical adsorption
- Nature of adsorbate: Readily liquefiable gases such as HCl, etc. are more. easily adsorbed by and adsorbent than the permanent gases etc.
- Nature of absorbent: - Activated charcoal is a better adsorbent than transitional metals.
Specific area of adsorbent-The greater the specific area of adsorbent the greater will be the extent of adsorption. Extent of adsorption state of sub- division of adsorbent.
Pressure: - Extent of physical adsorption increases as the pressure of the gas increases, till a saturation point is reached.
Freundlich gave the following relationship between extent of adsorption
Where:
mass of adsorbate, of adsorbent and pressure :
and are constants, depends upon the nature of adsorbate as well as adsorbent.
(a) At low pressure: is directly proportional to the pressure
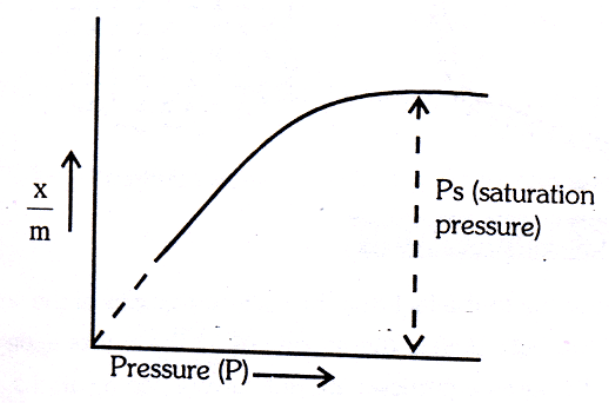
(b) At high pressure: - The extent of adsorption,
becomes independent of pressure i.e. a. PO
(c) At intermediate pressure: - will depend upon the power of
pressure which lies between 0 and 1
or (i)
On taking logarithm on both sides of
(i) We get
(ii)
Expression (i) and (ii) are called Freundlich adsorption isotherm.
Expression (ii) represents a straight line. The slope of the line will be while its intercept on the log
axis will be
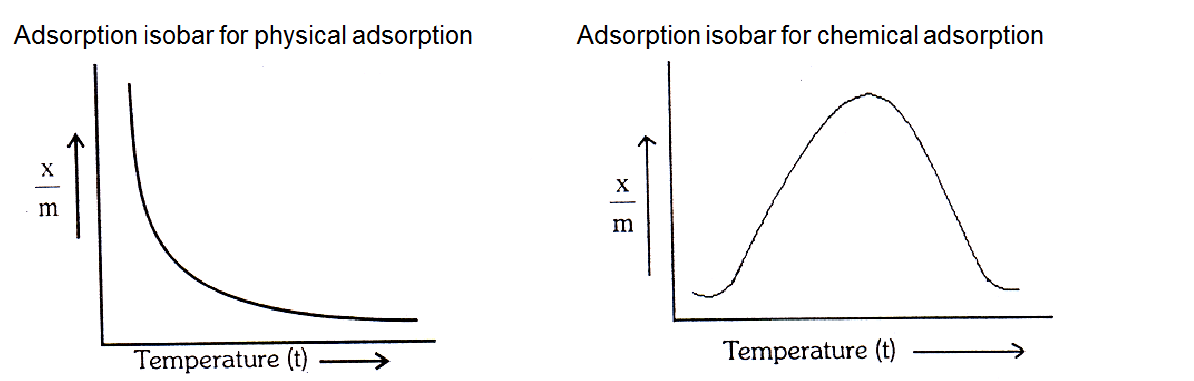
Temperature: Adsorption is accompanied by evolution of heat i.e. H is negative, so the rate of adsorption should decrease with rise in temperature. It is found to be so in case of physical adsorption. The effect of temperature is represented by an adsorption isobar.
Activation of adsorbent-An adsorbent can be activated either by heating or by bringing it in finely divided state, or by making its surface rough by rubbing. For example, charcoal is activated by heating it in vacuum at
Adsorption isobar - A graph between the amount of adsorbate adsorbed per gram of adsorbent and the temperature (t) at a constant pressure is called adsorption isobar.

Catalysis
The phenomena in which the rate of a reaction is altered (increased or decrease) by the presence of a substance (Catalyst) is known as catalysis.
Catalytic reactions are divided into two types.
(a) Homogeneous catalysis
(b) Heterogeneous catalysis
Homogeneous catalysis:
When the reactants and the catalyst are in the same physical state, i.e. in the same phase, it is called homogeneous catalysis. For example.
(i) Lead chamber process: - In this process for the manufacture of sulphuric acid NO (gas) is used as a catalyst.
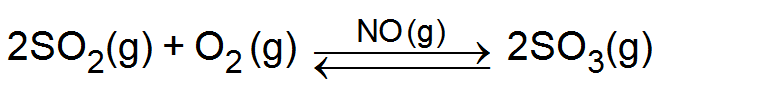
(ii) Inversion of cane sugar- In aqueous solution is catalysed by dilute acid (Hydrogen ions)
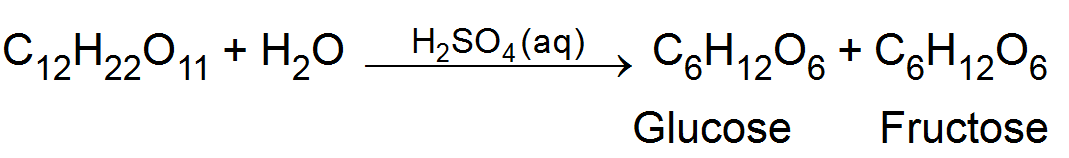 (b) Heterogeneous catalysis:
(b) Heterogeneous catalysis: When the catalyst and the reactants are not in the same physical state i.e. not in the same phase, it is called heterogeneous catalysis. for example.
(i) Decomposition of
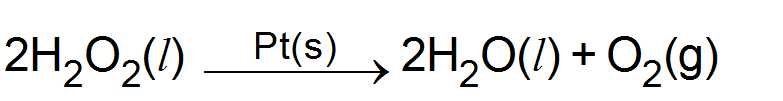
(ii) Haber process for
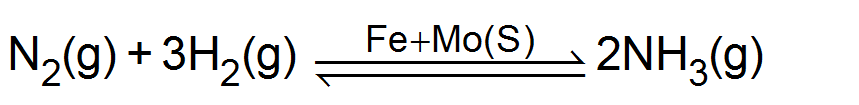
TYPES OF CATALYSIS
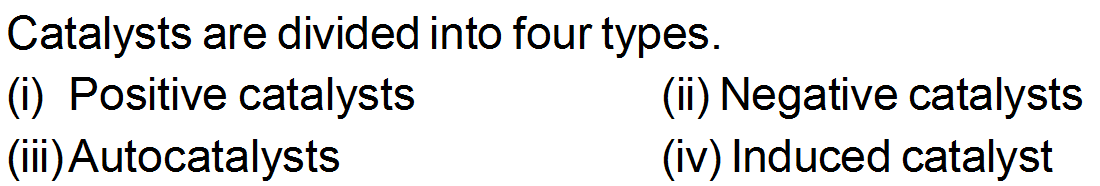 (i) Positive catalysts: -
(i) Positive catalysts: -The substance which increases the rate of a reaction is known as a positive catalyst. It decreases the energy of activation for the reaction. For example:
(ii) Negative catalysts: The substance which decreases the rate of chemical reaction is called negative catalyst or inhibitor. It increases the activation energy for the reaction. For example.
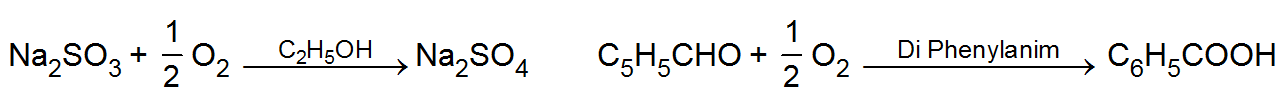 (iii) Auto catalysts: -
(iii) Auto catalysts: -When one of the products of the reaction begins to act as a catalyst, it is called auto-catalyst for example. In the initial stages the reaction is slow but as soon as the products come into existence the the reaction rate increases
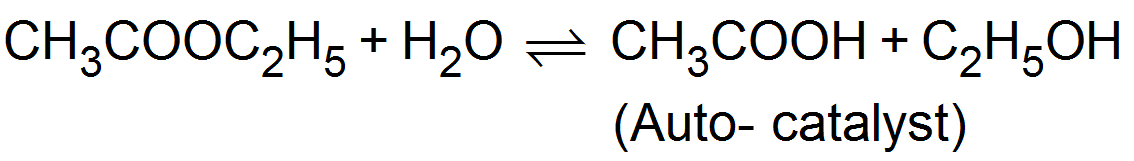 (iv) Induced catalyst:-
(iv) Induced catalyst:- When a chemical reaction enhances the rate of another chemical reaction it is called induced catalysis. For example:-
Soduim arsenite solution is not oxidised by air. If however, air is passed through a mixture of both of them undergo simultaneous oxidation. The oxidation of sodium sulphite, thus influences the oxidation of sodium arsenite.
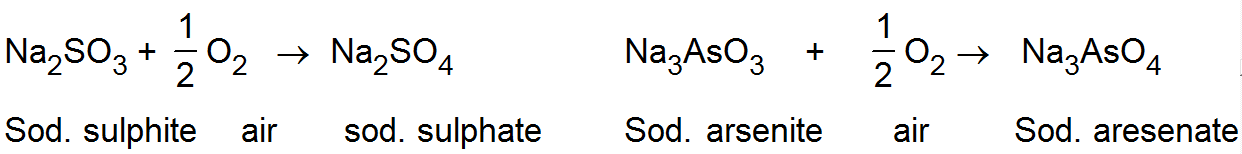 Promoters:
Promoters: Those substance which do not themselves act as catalysts but their presence increases the activity of a catalyst are called catalytic promoters or catalyst for a catalyst. Example: In the Haber process for the synthesis of ammonia, Fe is the catalyst while molybdenum (Mo) acts as promoter.
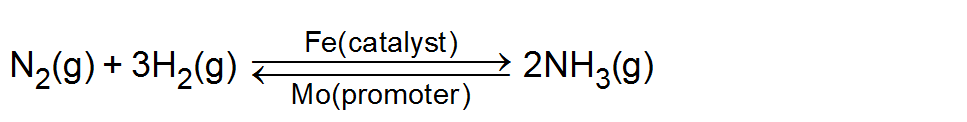 Catalytic Poison: -
Catalytic Poison: -The substance whose presence decreases or destroy the activity of a catalyst is called catalytic poison. For example:- Carbon monoxide or in hydrogen gas, acts as a poison for Fe catalyst in the Haber process for acts as poison for asbestos in contact process for
Inhibitors: - Those substances which retard rate of a chemical reaction are known as inhibitor. For example: glycerol or acetamide decrease the rate of decomposition of hydrogen peroxide.
General characteristics of catalysts:
(i) A catalyst remains unchanged in mass and chemical composition but change their physical state.
(ii) Only a very small amount of catalyst is sufficient to catalyse a reaction.
(iii) A catalyst does not initiate a reaction & does not controlled on chemical Rxn.
(iv) When a catalyst is a solid, it is usually more efficient where used in finely divided form.
(v) Generally, catalyst does not change the nature of products.
(vi) A catalyst does not change the equilibrium state of a reversible reaction but help to time achieve of equilibrium state. or position of equilibrium.
(vii) The catalysts are generally specific in nature.
(viii) Change rate constant of Rxn.
(ix) does not change free energy of Rxn.
THEORIES OF CATALYSIS
Intermediate compound formation theory: -This theory is explaining homogeneous catalysis mainly. According to this theory, the catalyst combines with one of the reactants to give an intermediate compound. This compound intermediately reacts with the other reactants and gives the product and regenerates the catalyst in its original form.
Thus, the reactants do not directly combine with each other, instead they react through the catalyst which provides an alternative pathway which involves lesser energy of activation.
For example: -The function of nitric oxide (NO) as a catalyst in the formation of is explained as follows.
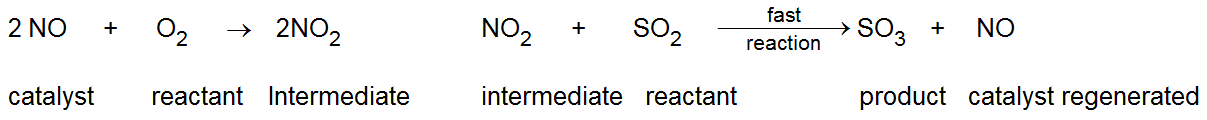 Adsorption theory: -
Adsorption theory: - This theory is explaining the heterogeneous catalysis. The role of a solid catalyst in enhancing the reaction rate is explained on the basis of this theory in the following steps.
(i) The reactant molecules are absorbed on the surface of the catalyst at adjacent points. Adsorption leads to higher concentration of the adsorbed reactant on the surface of a catalyst.
(ii) As adsorption is an exothermic process, the heat of adsorption provides the necessary activation energy for the chemical reaction to proceed.
(iii) The adsorbed reactant molecules are tied on the solid sold surface of the catalyst. The bonds between the atoms of chemisorption reactant molecules are weakened. The reactant molecules of sufficient energy combine together and with the surface of the catalyst to form surface activated complex.
This adsorbed activated complex is decomposed to form 'products as a definite faster rate.
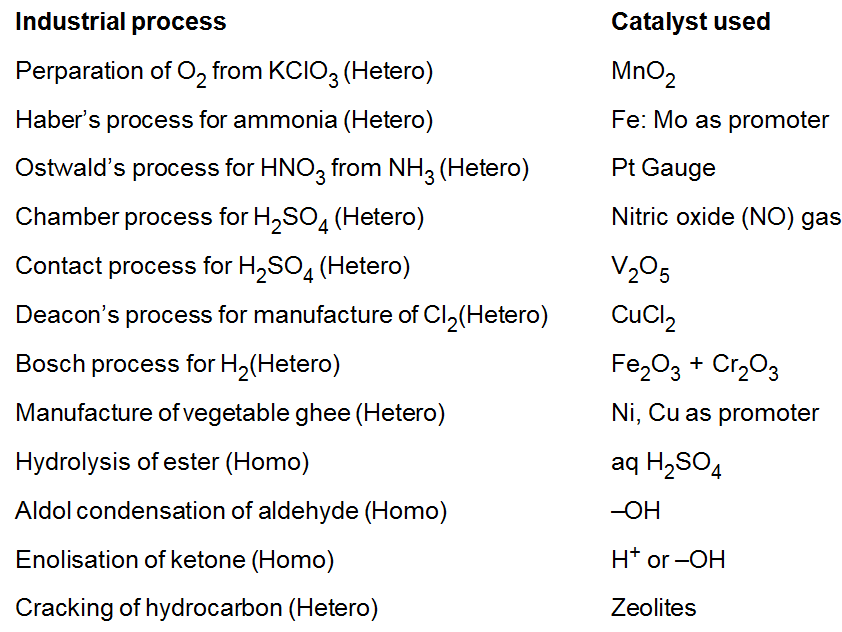
Catalysts in Industry: - Some of the important processes and their catalyst are given in below.
 Download eSaral App and ask your doubts for free at Discussion Forum.
Download eSaral App and ask your doubts for free at Discussion Forum.


 (b) At high pressure: - The extent of adsorption,
becomes independent of pressure i.e. a. PO
(c) At intermediate pressure: - will depend upon the power of
pressure which lies between 0 and 1
or (i)
On taking logarithm on both sides of
(i) We get
(ii)
Expression (i) and (ii) are called Freundlich adsorption isotherm.
Expression (ii) represents a straight line. The slope of the line will be while its intercept on the log
axis will be
Temperature: Adsorption is accompanied by evolution of heat i.e. H is negative, so the rate of adsorption should decrease with rise in temperature. It is found to be so in case of physical adsorption. The effect of temperature is represented by an adsorption isobar.
Activation of adsorbent-An adsorbent can be activated either by heating or by bringing it in finely divided state, or by making its surface rough by rubbing. For example, charcoal is activated by heating it in vacuum at
Adsorption isobar - A graph between the amount of adsorbate adsorbed per gram of adsorbent and the temperature (t) at a constant pressure is called adsorption isobar.
(b) At high pressure: - The extent of adsorption,
becomes independent of pressure i.e. a. PO
(c) At intermediate pressure: - will depend upon the power of
pressure which lies between 0 and 1
or (i)
On taking logarithm on both sides of
(i) We get
(ii)
Expression (i) and (ii) are called Freundlich adsorption isotherm.
Expression (ii) represents a straight line. The slope of the line will be while its intercept on the log
axis will be
Temperature: Adsorption is accompanied by evolution of heat i.e. H is negative, so the rate of adsorption should decrease with rise in temperature. It is found to be so in case of physical adsorption. The effect of temperature is represented by an adsorption isobar.
Activation of adsorbent-An adsorbent can be activated either by heating or by bringing it in finely divided state, or by making its surface rough by rubbing. For example, charcoal is activated by heating it in vacuum at
Adsorption isobar - A graph between the amount of adsorbate adsorbed per gram of adsorbent and the temperature (t) at a constant pressure is called adsorption isobar.


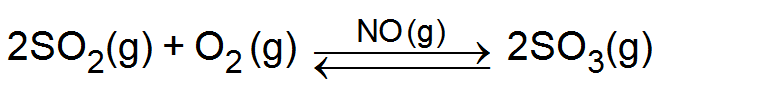 (ii) Inversion of cane sugar- In aqueous solution is catalysed by dilute acid (Hydrogen ions)
(ii) Inversion of cane sugar- In aqueous solution is catalysed by dilute acid (Hydrogen ions)
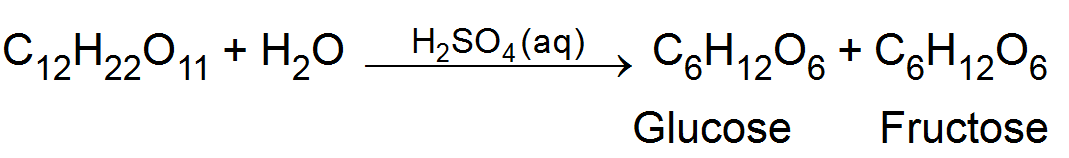 (b) Heterogeneous catalysis: When the catalyst and the reactants are not in the same physical state i.e. not in the same phase, it is called heterogeneous catalysis. for example.
(i) Decomposition of
(b) Heterogeneous catalysis: When the catalyst and the reactants are not in the same physical state i.e. not in the same phase, it is called heterogeneous catalysis. for example.
(i) Decomposition of
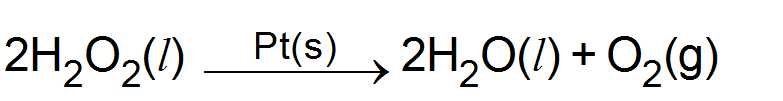 (ii) Haber process for
(ii) Haber process for
 (i) Positive catalysts: -The substance which increases the rate of a reaction is known as a positive catalyst. It decreases the energy of activation for the reaction. For example:
(ii) Negative catalysts: The substance which decreases the rate of chemical reaction is called negative catalyst or inhibitor. It increases the activation energy for the reaction. For example.
(i) Positive catalysts: -The substance which increases the rate of a reaction is known as a positive catalyst. It decreases the energy of activation for the reaction. For example:
(ii) Negative catalysts: The substance which decreases the rate of chemical reaction is called negative catalyst or inhibitor. It increases the activation energy for the reaction. For example.
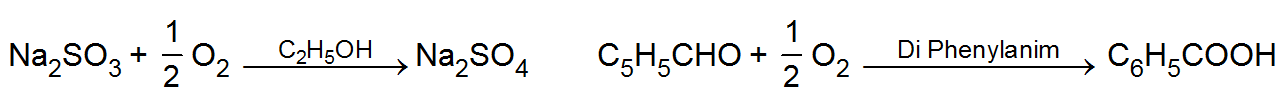 (iii) Auto catalysts: -When one of the products of the reaction begins to act as a catalyst, it is called auto-catalyst for example. In the initial stages the reaction is slow but as soon as the products come into existence the the reaction rate increases
(iii) Auto catalysts: -When one of the products of the reaction begins to act as a catalyst, it is called auto-catalyst for example. In the initial stages the reaction is slow but as soon as the products come into existence the the reaction rate increases
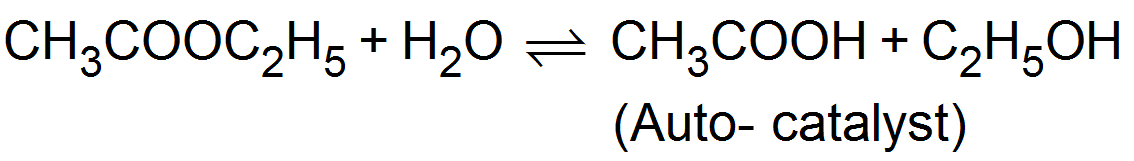 (iv) Induced catalyst:- When a chemical reaction enhances the rate of another chemical reaction it is called induced catalysis. For example:-
Soduim arsenite solution is not oxidised by air. If however, air is passed through a mixture of both of them undergo simultaneous oxidation. The oxidation of sodium sulphite, thus influences the oxidation of sodium arsenite.
(iv) Induced catalyst:- When a chemical reaction enhances the rate of another chemical reaction it is called induced catalysis. For example:-
Soduim arsenite solution is not oxidised by air. If however, air is passed through a mixture of both of them undergo simultaneous oxidation. The oxidation of sodium sulphite, thus influences the oxidation of sodium arsenite.
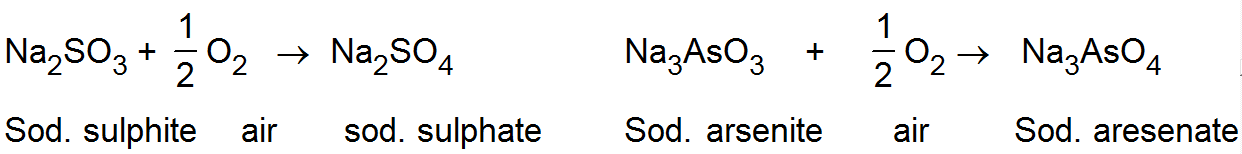 Promoters: Those substance which do not themselves act as catalysts but their presence increases the activity of a catalyst are called catalytic promoters or catalyst for a catalyst. Example: In the Haber process for the synthesis of ammonia, Fe is the catalyst while molybdenum (Mo) acts as promoter.
Promoters: Those substance which do not themselves act as catalysts but their presence increases the activity of a catalyst are called catalytic promoters or catalyst for a catalyst. Example: In the Haber process for the synthesis of ammonia, Fe is the catalyst while molybdenum (Mo) acts as promoter.
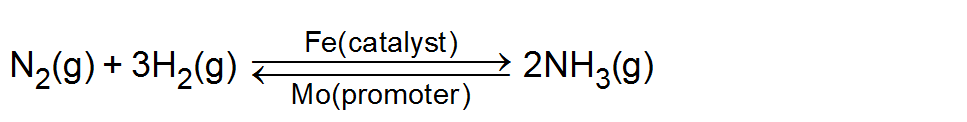 Catalytic Poison: -The substance whose presence decreases or destroy the activity of a catalyst is called catalytic poison. For example:- Carbon monoxide or in hydrogen gas, acts as a poison for Fe catalyst in the Haber process for acts as poison for asbestos in contact process for
Inhibitors: - Those substances which retard rate of a chemical reaction are known as inhibitor. For example: glycerol or acetamide decrease the rate of decomposition of hydrogen peroxide.
General characteristics of catalysts:
(i) A catalyst remains unchanged in mass and chemical composition but change their physical state.
(ii) Only a very small amount of catalyst is sufficient to catalyse a reaction.
(iii) A catalyst does not initiate a reaction & does not controlled on chemical Rxn.
(iv) When a catalyst is a solid, it is usually more efficient where used in finely divided form.
(v) Generally, catalyst does not change the nature of products.
(vi) A catalyst does not change the equilibrium state of a reversible reaction but help to time achieve of equilibrium state. or position of equilibrium.
(vii) The catalysts are generally specific in nature.
(viii) Change rate constant of Rxn.
(ix) does not change free energy of Rxn.
THEORIES OF CATALYSIS
Intermediate compound formation theory: -This theory is explaining homogeneous catalysis mainly. According to this theory, the catalyst combines with one of the reactants to give an intermediate compound. This compound intermediately reacts with the other reactants and gives the product and regenerates the catalyst in its original form.
Thus, the reactants do not directly combine with each other, instead they react through the catalyst which provides an alternative pathway which involves lesser energy of activation.
For example: -The function of nitric oxide (NO) as a catalyst in the formation of is explained as follows.
Catalytic Poison: -The substance whose presence decreases or destroy the activity of a catalyst is called catalytic poison. For example:- Carbon monoxide or in hydrogen gas, acts as a poison for Fe catalyst in the Haber process for acts as poison for asbestos in contact process for
Inhibitors: - Those substances which retard rate of a chemical reaction are known as inhibitor. For example: glycerol or acetamide decrease the rate of decomposition of hydrogen peroxide.
General characteristics of catalysts:
(i) A catalyst remains unchanged in mass and chemical composition but change their physical state.
(ii) Only a very small amount of catalyst is sufficient to catalyse a reaction.
(iii) A catalyst does not initiate a reaction & does not controlled on chemical Rxn.
(iv) When a catalyst is a solid, it is usually more efficient where used in finely divided form.
(v) Generally, catalyst does not change the nature of products.
(vi) A catalyst does not change the equilibrium state of a reversible reaction but help to time achieve of equilibrium state. or position of equilibrium.
(vii) The catalysts are generally specific in nature.
(viii) Change rate constant of Rxn.
(ix) does not change free energy of Rxn.
THEORIES OF CATALYSIS
Intermediate compound formation theory: -This theory is explaining homogeneous catalysis mainly. According to this theory, the catalyst combines with one of the reactants to give an intermediate compound. This compound intermediately reacts with the other reactants and gives the product and regenerates the catalyst in its original form.
Thus, the reactants do not directly combine with each other, instead they react through the catalyst which provides an alternative pathway which involves lesser energy of activation.
For example: -The function of nitric oxide (NO) as a catalyst in the formation of is explained as follows.
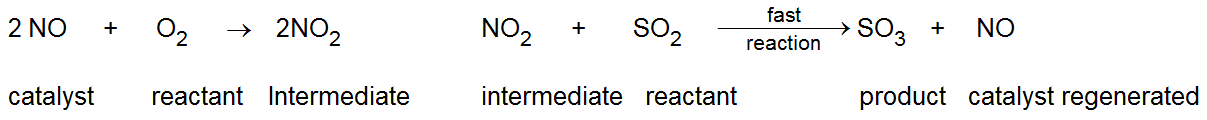 Adsorption theory: - This theory is explaining the heterogeneous catalysis. The role of a solid catalyst in enhancing the reaction rate is explained on the basis of this theory in the following steps.
(i) The reactant molecules are absorbed on the surface of the catalyst at adjacent points. Adsorption leads to higher concentration of the adsorbed reactant on the surface of a catalyst.
(ii) As adsorption is an exothermic process, the heat of adsorption provides the necessary activation energy for the chemical reaction to proceed.
(iii) The adsorbed reactant molecules are tied on the solid sold surface of the catalyst. The bonds between the atoms of chemisorption reactant molecules are weakened. The reactant molecules of sufficient energy combine together and with the surface of the catalyst to form surface activated complex.
This adsorbed activated complex is decomposed to form 'products as a definite faster rate.
Catalysts in Industry: - Some of the important processes and their catalyst are given in below.
Adsorption theory: - This theory is explaining the heterogeneous catalysis. The role of a solid catalyst in enhancing the reaction rate is explained on the basis of this theory in the following steps.
(i) The reactant molecules are absorbed on the surface of the catalyst at adjacent points. Adsorption leads to higher concentration of the adsorbed reactant on the surface of a catalyst.
(ii) As adsorption is an exothermic process, the heat of adsorption provides the necessary activation energy for the chemical reaction to proceed.
(iii) The adsorbed reactant molecules are tied on the solid sold surface of the catalyst. The bonds between the atoms of chemisorption reactant molecules are weakened. The reactant molecules of sufficient energy combine together and with the surface of the catalyst to form surface activated complex.
This adsorbed activated complex is decomposed to form 'products as a definite faster rate.
Catalysts in Industry: - Some of the important processes and their catalyst are given in below.

