hey, do you want to learn about the Classification of solids in terms of the forbidden energy gap? If yes. Then you are at the right place.
Classification of solids in terms of the forbidden energy gap
The width of the forbidden energy gap between the valence band and the conduction band distinguishes conductors, semiconductors, and insulators from each other. 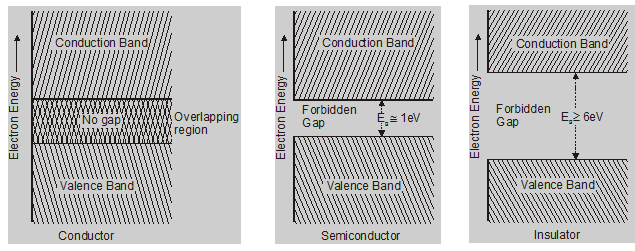
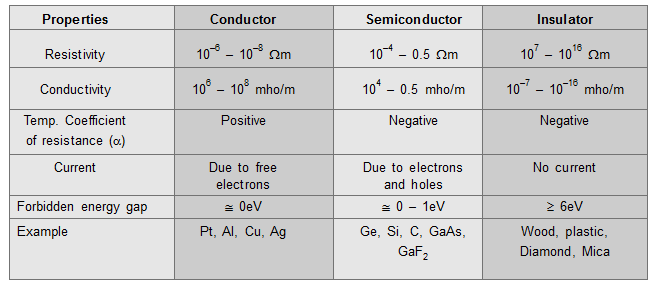
Comparison between conductor, semiconductor, and insulator:
Difference between Valence, Conduction, and Forbidden Band
Valence band:
This band is never empty. VB may be partially or completely filled with electrons. VB electrons are not capable of gaining energy from the external electric fields. Therefore, the electrons of VB also not contribute to the electric current.
Conduction band:
Electrons are rarely present. CB either empty or partially filled with electrons. CB electrons can gain energy from the external electric field. Electrons in this band contribute to the electric current.
Forbidden energy gap:
Electrons are not found in this band. FEB is completely empty. The minimum energy required for shifting electrons from the valence band to the conduction band is called band gap ($\left(E_{g}\right)$).
So, that's all from this article. I hope you get the idea about the Classification of the solids according to the Forbidden energy gaps. If you found this article informative then please share it with your friends. If you have any confusion related to this topic, then you can ask in the comments section down below.
For a better understanding of this chapter, please check the detailed notes of Electronics. To watch Free Learning Videos on physics by Saransh Gupta sir Install the eSaral App.
