Learn about Coagulation of Colloids, Electrophoresis, water in oil emulsion, oil in water emulsion, Associated Colloids and more. Get to know about the Colloid Protection here.
Charge on Colloidal Particles
Helmholtz Electrical Double Layer
Electrophoresis
Electro-osmosis
Coagulation of Colloids & its methods
Hardy Schulze Law
Protective Colloids
Emulsions
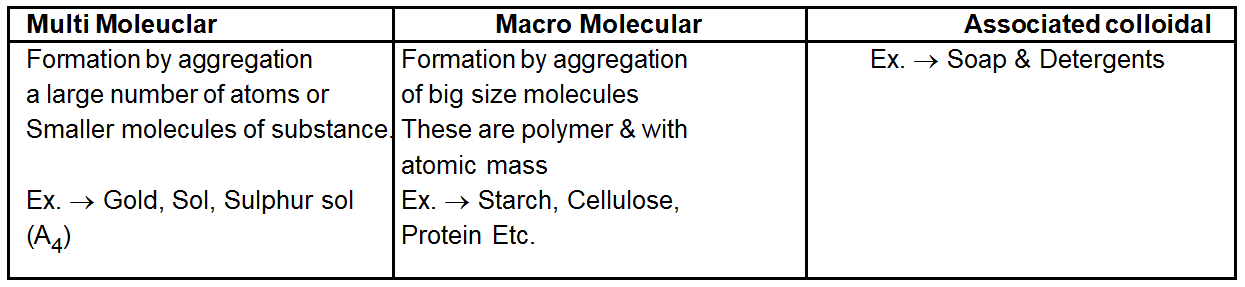
Associated Colloids
Gels
 The combination of the compact and diffused layer is referred as the stern double layer. The diffused layer is only loosely attached to the particles surface and moves in the opposite direction under an applied electric field. The potential difference between., the fixed layer and the diffused layer of opposite charge is called electro kinetic potential or zeta potential.
The combination of the compact and diffused layer is referred as the stern double layer. The diffused layer is only loosely attached to the particles surface and moves in the opposite direction under an applied electric field. The potential difference between., the fixed layer and the diffused layer of opposite charge is called electro kinetic potential or zeta potential.
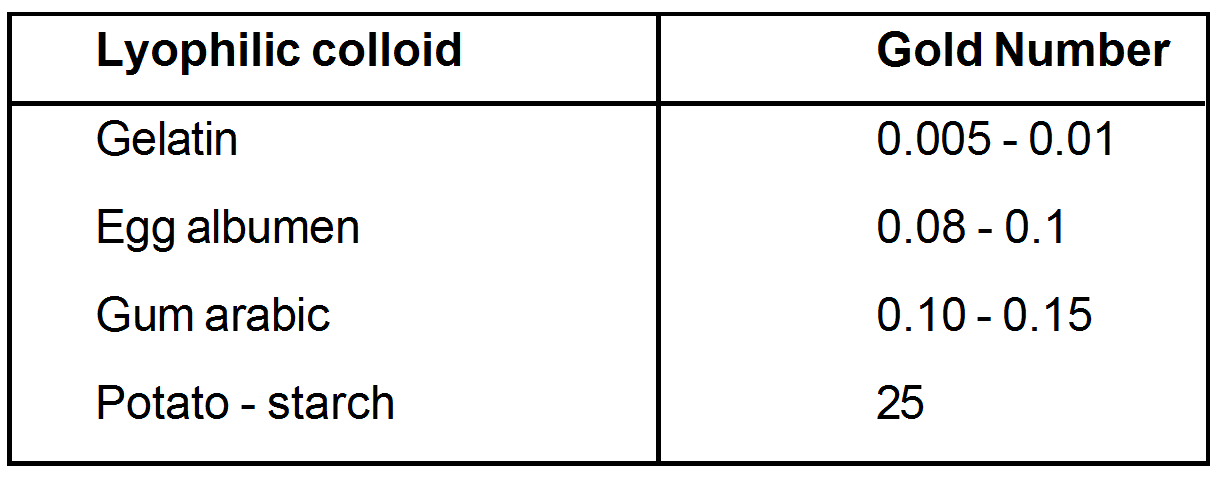
 The Lyophilic colloids differ in their protective power. The protective power is measured in terms of "Gold number introduced by Zsigmondy.
The number of milligrams of a hydrophilic colloid that will just prevent the precipitation of 10 of gold solon addition of 1 of 10 solution is known as gold number of that protector (Lyophilic colloid) On the onset of precipitation of the gold sol is indicated by a colour change from red to blue when the particle size just increases.
The smaller the gold number of a protective Lyophilic colloid, greater is its protection power.
Gold Number of some Lyophilic colloids
The Lyophilic colloids differ in their protective power. The protective power is measured in terms of "Gold number introduced by Zsigmondy.
The number of milligrams of a hydrophilic colloid that will just prevent the precipitation of 10 of gold solon addition of 1 of 10 solution is known as gold number of that protector (Lyophilic colloid) On the onset of precipitation of the gold sol is indicated by a colour change from red to blue when the particle size just increases.
The smaller the gold number of a protective Lyophilic colloid, greater is its protection power.
Gold Number of some Lyophilic colloids
 Protection Capacity
Gelatine and starch have the maximum and minimum protective powers.
Protection Capacity
Gelatine and starch have the maximum and minimum protective powers.
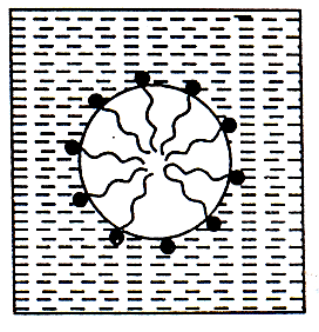
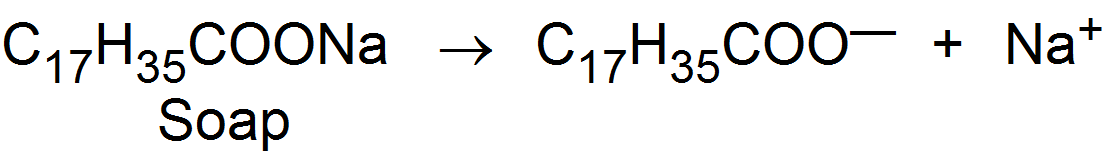 The negative ions aggregate to form a micelle of colloidal size. The negative ion has a long Hydrocarbon chain and a polar groupin water it directed towards the center while the soluble polar head is on the surface in contact with water. The charge on the micelle is responsible for the stability of this system.
The negative ions aggregate to form a micelle of colloidal size. The negative ion has a long Hydrocarbon chain and a polar groupin water it directed towards the center while the soluble polar head is on the surface in contact with water. The charge on the micelle is responsible for the stability of this system.
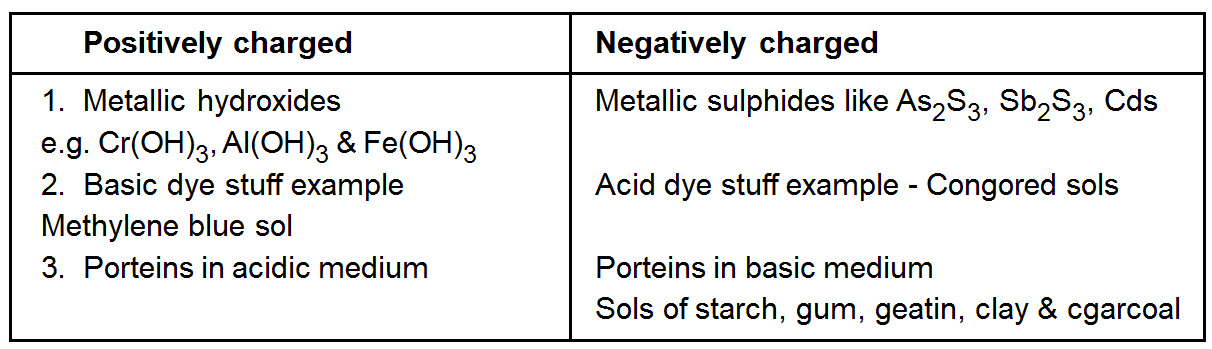
Charge on colloidal particles
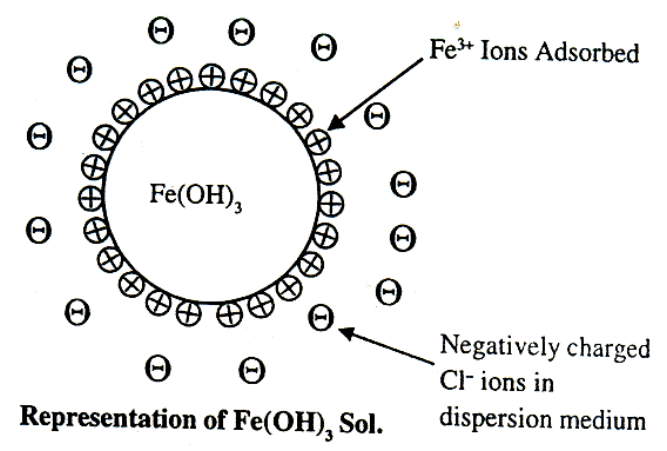
Colloidal particles always carry an electric charge. The mutual forces of repulsion between similarly charged particles prevent them from aggregating and settling under the action of gravity. This gives stability to the sole A list of common sols with the type of change on their particles is given ahead.

Helmholtz Electrical Double Layer
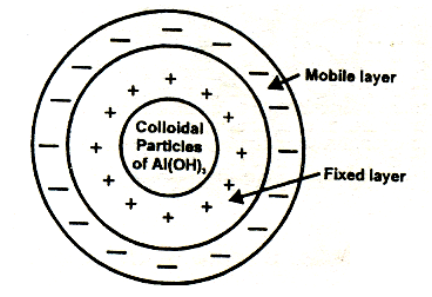
The surface of a colloidal particle acquires a type by selective adsorption of a layer of positive ions around it. This layer attracts counter ions from the medium which form a second layer of -ve charges. The combination of the two layers of +ve and-ve charges around the sol particle was called Helmholtz double layer. According to modern view, the first layer of ions is firmly held and is termed fixed layer while the second layer is mobile which is termed as diffused layer. The combination of the compact and diffused layer is referred as the stern double layer. The diffused layer is only loosely attached to the particles surface and moves in the opposite direction under an applied electric field. The potential difference between., the fixed layer and the diffused layer of opposite charge is called electro kinetic potential or zeta potential.
The combination of the compact and diffused layer is referred as the stern double layer. The diffused layer is only loosely attached to the particles surface and moves in the opposite direction under an applied electric field. The potential difference between., the fixed layer and the diffused layer of opposite charge is called electro kinetic potential or zeta potential.
Electrophoresis Meaning
If electric potential is applied across two platinum electrodes dipping in a colloidal solution, the colloidal particles move towards one or the other electrode, due to charge them, The movement of sol particles under an applied electric potential is called "Electrophoresis", Depending upon the direction of movement of particles towards cathode, or anode electrophoresis can be called "cataphoresis' or 'Anaphoresis".Electrophoresis provides and experimental proof to show that the colloidal particles are charged particles.Electro-osmosis
The medium will move in opposite direction to the dispersed phase under the influence of applied electric potential. The movement of dispersion medium under the influence of applied potential is known as 'Electro - osmosis'.Coagulation of Colloids
We know that the stability of a lyophobic sol is due to the adsorption of positive or negative ions by the dispersed particles. The repulsion forces between the charged particles do not allow them to settle. If somehow, the charge is removed there is nothing to keep the particles apart from each other. In such cases they aggregate or flocculate and settle down under the action of gravity the flocculation and settling down of the discharged sol particles is called coagulation or the precipitation can be brought about in four waysMethods of Coagulation
(a) By addition of electrolyte. (b) By electrophoresis. (c) By mixing two oppositely charged sols. (d) By boiling.(a) By addition of electrolytes
When an electrolyte is added in excess to a sol, then the electrolyte furnishes both the type of ions in solution. The oppositely charged ions get adsorbed on the surface of colloidal particles this causes neutralization and there by the size and mass of colloidal particle increases and it becomes a suspension particle. Due to greater volume and greater mass these suspension particles settle down i.e. they coagulate. The ion responsible for neutralization of charge on the particle is called the flocculating ion,Hardy Schulze Law
states that the precipitating effect of an ion on dispersed phase of opposite charge increases with the valence of the ion. The higher the valency of the flocculating ion, the greater is its precipitating power. Thus, for the precipitation of solutions. Similarly for precipitating sol (positive) the precipitating power of and is in the orderFLOCCULATION VALUE
The minimum ,concentration of an electrolyte in milli moles per litre required to cause precipitation of a sol in 2 hours is called FLOCCULATION VALUE. The smaller the flocculating value, the higher will be the coagulating power of the ion.(b) By Electrophoresis
During electrophoresis the charged sol particles migrate towards the electrode of opposite sign. There they deposit their charge and then get coagulated (As the neutral particles can aggregate and change to suspension particles.(c) By mixing two oppositely charged sols-
The neutral coagulation of two sols of opposite charge can be affected by mixing them. For e.g. (positive) sol and Arsenious sulphide (negative sol) when mixed join and coagulate.(d) By boiling
Sols such as sulphur and silver halides disperse in water disperse in water, get coagulated when boiled due to increased collisions between sol particles and water molecules, which removes the adsorbed charged layer from the sol and therefore the sol particles settle down.Protective Colloids : Protection or Protective action.
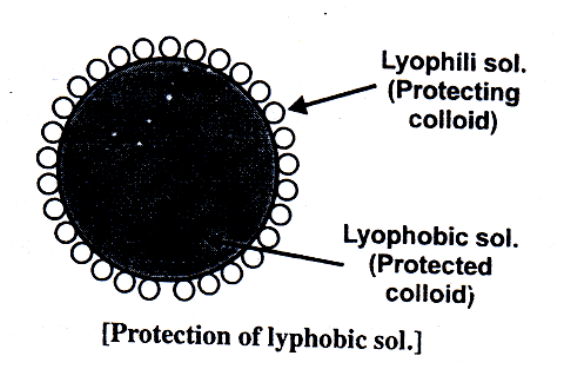
Lyophobic sols are readily precipitated by small amounts of electrolytes. However, these sols often stabilized by the addition of Lyophilic sols. The property of Lyophilic soles to prevent the precipitation or coagulation of a lyophobic salt is called protection. The Lyophilic sol used to protect a lyophobic sol from precipitation is referred to as a protective colloid. Lyophilic sols form a thin layer around lyophobic sol or around the ions furnished by electrolyte and therefore the coagulation cannot take place (as the size does not increase much). Gelatine, Albumen, Gum Arabia, Potato Starch are some of the examples of protective colloids. The Lyophilic colloids differ in their protective power. The protective power is measured in terms of "Gold number introduced by Zsigmondy.
The number of milligrams of a hydrophilic colloid that will just prevent the precipitation of 10 of gold solon addition of 1 of 10 solution is known as gold number of that protector (Lyophilic colloid) On the onset of precipitation of the gold sol is indicated by a colour change from red to blue when the particle size just increases.
The smaller the gold number of a protective Lyophilic colloid, greater is its protection power.
Gold Number of some Lyophilic colloids
The Lyophilic colloids differ in their protective power. The protective power is measured in terms of "Gold number introduced by Zsigmondy.
The number of milligrams of a hydrophilic colloid that will just prevent the precipitation of 10 of gold solon addition of 1 of 10 solution is known as gold number of that protector (Lyophilic colloid) On the onset of precipitation of the gold sol is indicated by a colour change from red to blue when the particle size just increases.
The smaller the gold number of a protective Lyophilic colloid, greater is its protection power.
Gold Number of some Lyophilic colloids
 Protection Capacity
Gelatine and starch have the maximum and minimum protective powers.
Protection Capacity
Gelatine and starch have the maximum and minimum protective powers.
Emulsions
These are liquid-liquid colloidal systems. There are two types Emulsions (i) Oil dispersed in water (o/w types) (ii) Water Dispersed in oil (w/o types) water in oil emulsion Examples Oil in water Emulsion In the first type water acts dispersion medium examples of this type of emulsions are milk and vanishing cream. In milk, liquid fat is dispersed in water. In the second system oil acts as dispersion medium common examples of this type are butter and cream. Emulsions of oil and water are unstable and sometimes they separate into two layers on standing. For stabilization an of an emulsion, a third component called emulsifying agent is usually added. The emulsifying agent form an inter facial film between suspended particles and the medium. The Principal agent for of emulsions are proteins, gums, soaps, etc. for w/o emulsion the principal emulsifying agents are heavy metal salts of fatty acids, long chain alcohols etc.Associated Colloids [Micelles]
Substances whose molecules aggregates to form particles of colloidal dimensions are called associated colloids. The molecules of soaps and detergents are usually smaller than the colloidal particles. However, in concentrated solutions, these molecules associated and form aggregates of colloidal size. These aggregates of soaps or detergent molecules are called micelles. Soaps and detergents are strong electrolytes and gives ions when dissolved in water
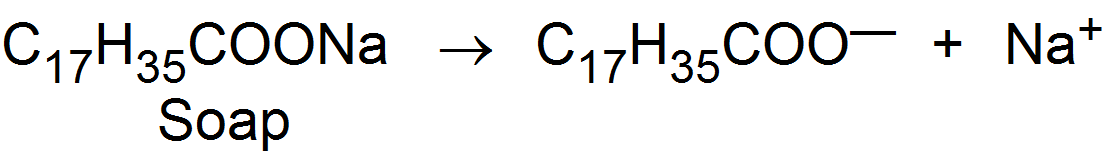 The negative ions aggregate to form a micelle of colloidal size. The negative ion has a long Hydrocarbon chain and a polar groupin water it directed towards the center while the soluble polar head is on the surface in contact with water. The charge on the micelle is responsible for the stability of this system.
The negative ions aggregate to form a micelle of colloidal size. The negative ion has a long Hydrocarbon chain and a polar groupin water it directed towards the center while the soluble polar head is on the surface in contact with water. The charge on the micelle is responsible for the stability of this system.
Gels
A gel is a jelly like colloidal system in which a liquid is dispersed in a solid medium. Gels may be classified into two types (a) Elastic gels- These are those which possesses the property of elasticity. They change their shape on applying force and return to original shape when the force is removed. Gelatine, starch and soaps are examples substances which form elastic gels. (b) Non- elastic gels- These are the gels which are rigid ego Silica gel. These are prepared by appropriate chemical action. Thus, silica gel is produced by adding concentrated Hydrochloric Acid to sodium silicate solution of the correct concentration.
Click here to Continue. . .
