JEE Main Previous Year Papers Questions of Chemistry With Solutions are available at eSaral.
Simulator
Previous Years AIEEE/JEE Mains Questions
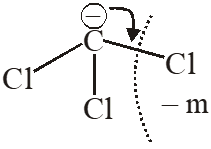
Q. Arrange the carbanions, $\left(\mathrm{CH}_{3}\right)_{3} \overline{\mathrm{C}}, \overline{\mathrm{C}} \mathrm{Cl}_{3},\left(\mathrm{CH}_{3}\right)_{2} \overline{\mathrm{C}} \mathrm{H}, \mathrm{C}_{6} \mathrm{H}_{5} \overline{\mathrm{C}} \mathrm{H}_{2}$ , in order of their
decreasing stability
[AIEEE-2009]
Ans. (1)

Q. The non aromatic compound among the following is :-
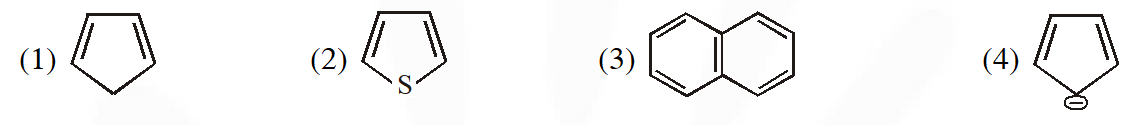 [AIEEE-2011]
[AIEEE-2011]
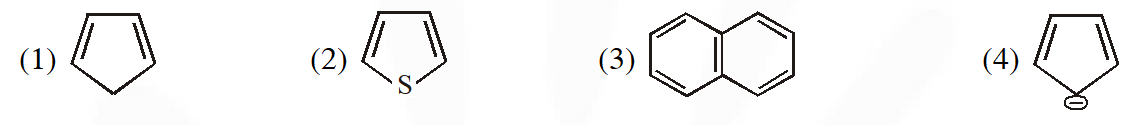 [AIEEE-2011]
[AIEEE-2011]
Ans. (1)
Q. ortho-Nitrophenol is less soluble in water than p– and m– Nitrophenols because :-
(1) Melting point of o–Nitrophenol is lower than those of m– and p– isomers
(2) o–Nitrophenol is more volatile in steam than those of m– and p– isomers
(3) o–Nitrophenol shows Intramolecular H–bonding
(4) o–Nitrophenol shows Intermolecular H–bonding
[AIEEE-2012]
Ans. (3)
Q. Which of the following compounds are antiaromatic :-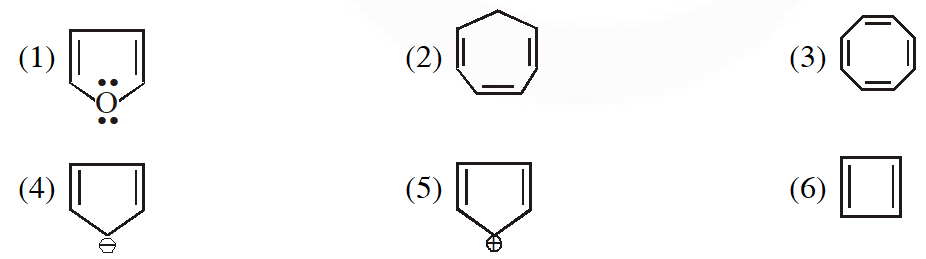 (1) (1) and (6) (2) (2) and (5) (3) (1) and (5) (4) (5) and (6)
[AIEEE-2012(Online)]
(1) (1) and (6) (2) (2) and (5) (3) (1) and (5) (4) (5) and (6)
[AIEEE-2012(Online)]
 (1) (1) and (6) (2) (2) and (5) (3) (1) and (5) (4) (5) and (6)
[AIEEE-2012(Online)]
(1) (1) and (6) (2) (2) and (5) (3) (1) and (5) (4) (5) and (6)
[AIEEE-2012(Online)]
Ans. (4)
Q. Among the following the molecule with the lowest dipole moment is :-
(1) $\mathrm{CHCl}_{3}$
(2) $\mathrm{CH}_{2} \mathrm{Cl}_{2}$
(3) $\mathrm{CCl}_{4}$
(4) $\mathrm{CH}_{3} \mathrm{Cl}$
[AIEEE-2012(Online)]
Ans. (3)
Q. The order of stability of the following carbocations
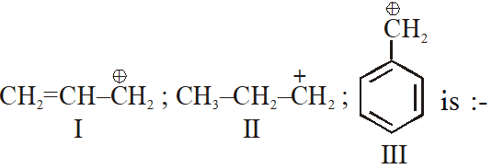 (1) III > II > I
(2) II > III > I
(3) I > II > III
(4) III > I > II
[JEE-MAIN-2013]
(1) III > II > I
(2) II > III > I
(3) I > II > III
(4) III > I > II
[JEE-MAIN-2013]
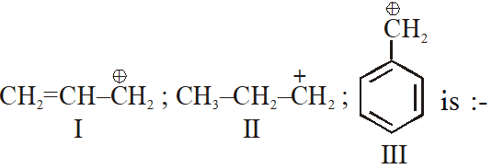 (1) III > II > I
(2) II > III > I
(3) I > II > III
(4) III > I > II
[JEE-MAIN-2013]
(1) III > II > I
(2) II > III > I
(3) I > II > III
(4) III > I > II
[JEE-MAIN-2013]
Ans. (4)
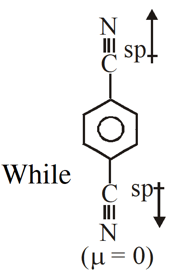
Q. For which of the following molecule significant $\mu^{1} 0$
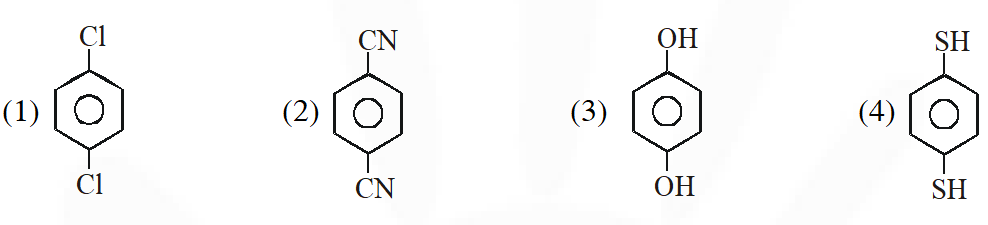 (1) Only (3) (2) (3) and (4) (3) Only (1) (4) (1) and (2)
[JEE-MAIN-2014]
(1) Only (3) (2) (3) and (4) (3) Only (1) (4) (1) and (2)
[JEE-MAIN-2014]
 (1) Only (3) (2) (3) and (4) (3) Only (1) (4) (1) and (2)
[JEE-MAIN-2014]
(1) Only (3) (2) (3) and (4) (3) Only (1) (4) (1) and (2)
[JEE-MAIN-2014]
Ans. (2)
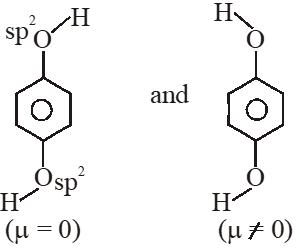 So dipole moment per mole (containing $\mathrm{N}_{\mathrm{A}}$ molecules) is non
zero. Some concept applicable for option (4) .
So dipole moment per mole (containing $\mathrm{N}_{\mathrm{A}}$ molecules) is non
zero. Some concept applicable for option (4) .
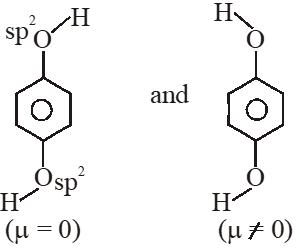 So dipole moment per mole (containing $\mathrm{N}_{\mathrm{A}}$ molecules) is non
zero. Some concept applicable for option (4) .
So dipole moment per mole (containing $\mathrm{N}_{\mathrm{A}}$ molecules) is non
zero. Some concept applicable for option (4) .

Q. The correct order of increasing basicity of the given conjugate base $\left(\mathrm{R}=\mathrm{CH}_{3}\right.$) is :-
$(1) \mathrm{RCO} \overline{\mathrm{O}}<\mathrm{HC} \equiv \overline{\mathrm{C}}<\overline{\mathrm{N}} \mathrm{H}_{2}<\overline{\mathrm{R}}$
(2) $\mathrm{RCO} \overline{\mathrm{O}}<\mathrm{HC} \equiv \overline{\mathrm{C}}<\overline{\mathrm{R}}<\overline{\mathrm{N}} \mathrm{H}_{2}$
(3) $\quad \overline{\mathrm{R}}<\mathrm{HC} \equiv \overline{\mathrm{C}}<\mathrm{RCO} \overline{\mathrm{O}}<\overline{\mathrm{N}} \mathrm{H}_{2}$
(4) $\mathrm{RCO} \overline{\mathrm{O}}<\overline{\mathrm{N} H}_{2}<\mathrm{HC} \equiv \overline{\mathrm{C}}<\overline{\mathrm{R}}$
[AIEEE-2010]
Ans. (1)
Basic strength $\propto \frac{1}{\text { Stability of conjugate base }}$
Q. The strongest acid amongst the following compounds is ?
(1) $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}(\mathrm{Cl}) \mathrm{CO}_{2} \mathrm{H}$
(2) $\mathrm{ClCH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{COOH}$
(3) $\mathrm{CH}_{3} \mathrm{COOH}$
(4) HCOOH
[AIEEE-2011]
Ans. (1)
(Acidity a –I effect)
Q. The correct order of acid strength of the following compounds :-
A. Phenol B. p-Cresol C. m-Nitrophenol D. p- Nitrophenol
(1) C > B > A > D (2) D > C > A > B
(3) B > D > A > C (4) A > B > D > C
[AIEEE-2011]
Ans. (2)
Q. In the following compounds :
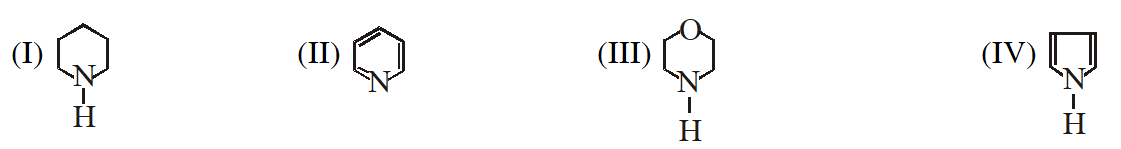 the order of basicity is as follows :
(1) IV > III > II > I
(2) II > III > I > IV
(3) I > III > II > IV
(4) III > I > II > IV
[JEE(Main)-2012]
the order of basicity is as follows :
(1) IV > III > II > I
(2) II > III > I > IV
(3) I > III > II > IV
(4) III > I > II > IV
[JEE(Main)-2012]
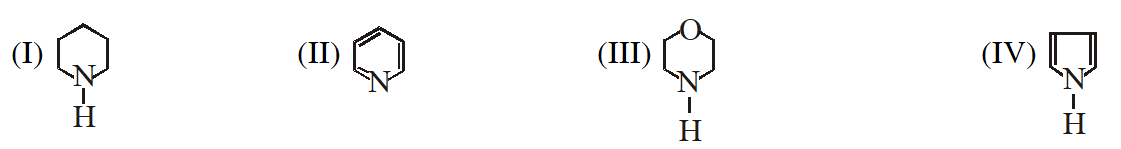 the order of basicity is as follows :
(1) IV > III > II > I
(2) II > III > I > IV
(3) I > III > II > IV
(4) III > I > II > IV
[JEE(Main)-2012]
the order of basicity is as follows :
(1) IV > III > II > I
(2) II > III > I > IV
(3) I > III > II > IV
(4) III > I > II > IV
[JEE(Main)-2012]
Ans. (3)
Q. The most basic compound among the following is :-
(1) Acetanilide
(2) Benzylamine
(3) p-Nitro aniline
(4) Aniline
[JEE(Main)-2012]
Ans. (2)
Basicity $\propto \frac{1}{\text { Resonance of lone pair e }}$
Q. The order of basicity of amines in gaseous state is :-
(1) $3^{\circ}>2^{\circ}>\mathrm{NH}_{3}>1^{\circ}$
(2) $1^{\circ}>2^{\circ}>3^{\circ}>\mathrm{NH}_{3}$
(3) $\mathrm{NH}_{3}>1^{\circ}>2^{\circ}>3^{\circ}$
(4) $3^{\circ}>2^{\circ}>1^{\circ}>\mathrm{NH}_{3}$
[JEE(Main)-2013]
Ans. (4)
Basicity $\propto+I$ effect
Q. Arrange the following compounds in order of decreasing acidity :
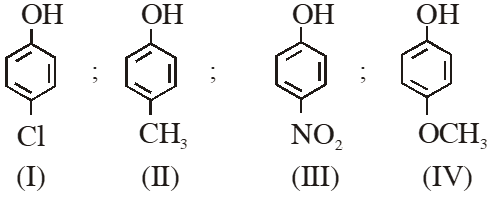 (1) II > IV > I > III
(2) I > II > III > IV
(3) III > I > II > IV
(4) IV > III > I > II
[JEE(Main)-2013]
(1) II > IV > I > III
(2) I > II > III > IV
(3) III > I > II > IV
(4) IV > III > I > II
[JEE(Main)-2013]
 (1) II > IV > I > III
(2) I > II > III > IV
(3) III > I > II > IV
(4) IV > III > I > II
[JEE(Main)-2013]
(1) II > IV > I > III
(2) I > II > III > IV
(3) III > I > II > IV
(4) IV > III > I > II
[JEE(Main)-2013]
Ans. (3)
Acidity $\propto-\mathrm{I} /-\mathrm{R} \propto \frac{1}{+\mathrm{I} /+\mathrm{R}}$
Q. The conjugate base of hydrazoic acid is :-
(1) $\mathrm{HN}_{3}^{-}$
(2) $\mathrm{N}_{3}^{-}$
(3) $\mathrm{N}_{2}^{-}$
(4) $\mathrm{N}^{-3}$
[JEE(Main)-2014]
Ans. (2)
$\left(\mathrm{HN}_{3} \longrightarrow \mathrm{N}_{3}+\mathrm{H}^{+}\right)$
Q. Which one of the following compounds will not be soluble in sodium bicarbonate ?
(1) Benzene sulphonic acid
(2) Benzoic acid
(3) o-Nitrophenol
(4) 2, 4, 6 - Trinitrophenol
[JEE(Main)-2014]
Ans. (2)
Due to ortho effect
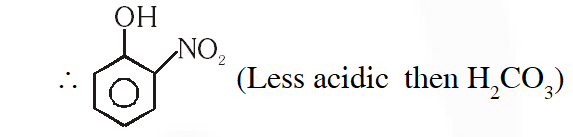
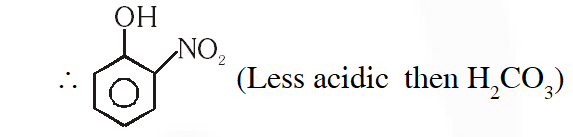
Q. Considering the basic strength of amines in aqueous solution, which one has
the smallest $\mathrm{pK}_{\mathrm{b}}$ value ?
(1) $\left(\mathrm{CH}_{3}\right)_{3} \mathrm{N}$
(2) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}$
(3) $\left(\mathrm{CH}_{3}\right)_{2} \mathrm{NH}$
(4) $\mathrm{CH}_{3} \mathrm{NH}_{2}$
[JEE(Main)-2014]
Ans. (3)
$\begin{aligned} \therefore \text { Due to solvation and inductive effect } \\ 2^{\circ}>1^{\circ}>3^{\circ}>0^{\circ} & \mathrm{R}=-\mathrm{CH}_{3} \end{aligned}$
Q. Among the following oxoacids, the correct decreasing order of acid strength is :
(1) $\mathrm{HClO}_{4}>\mathrm{HClO}_{3}>\mathrm{HClO}_{2}>\mathrm{HOCl}$
(2) $\mathrm{HClO}_{2}>\mathrm{HClO}_{4}>\mathrm{HClO}_{3}>\mathrm{HOCl}$
(3) $\mathrm{HOCl}>\mathrm{HClO}_{2}>\mathrm{HClO}_{3}>\mathrm{HClO}_{4}$
(4) $\mathrm{HClO}_{4}>\mathrm{HOCl}>\mathrm{HClO}_{2}>\mathrm{HClO}_{3}$
[JEE(Main)-2014]
Ans. (1)
$\therefore$ Acidic $\propto$ more E.R.S. of conjugate base
Q. The increasing order of basicity of the following compounds is :
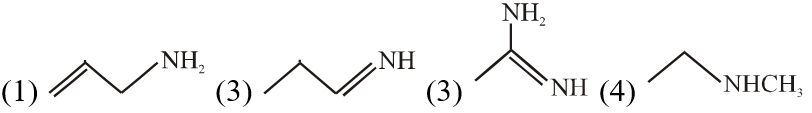 (1) (1) < (1) < (3) < (4)
(2) (1) < (1) < (4) < (3)
(3) (4) < (2) < (1) < (3)
(4) (1) < (2) < (3) < (3)
[JEE(Main)-2018]
(1) (1) < (1) < (3) < (4)
(2) (1) < (1) < (4) < (3)
(3) (4) < (2) < (1) < (3)
(4) (1) < (2) < (3) < (3)
[JEE(Main)-2018]
 (1) (1) < (1) < (3) < (4)
(2) (1) < (1) < (4) < (3)
(3) (4) < (2) < (1) < (3)
(4) (1) < (2) < (3) < (3)
[JEE(Main)-2018]
(1) (1) < (1) < (3) < (4)
(2) (1) < (1) < (4) < (3)
(3) (4) < (2) < (1) < (3)
(4) (1) < (2) < (3) < (3)
[JEE(Main)-2018]
Ans. (2)
Order of base nature depends on electron donation tendency.
In compound nitrogen is sp2 hybridized so least basic among all given compound.
compound
nitrogen is sp2 hybridized so least basic among all given compound.
compound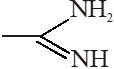 is very strong nitrogeneous organic base as lone pair of one nitrogen
delocalize in resonance and make another nitrogen negativly charged and conjugate acid have
two equivalent resonating structure.
Thus it is most basic in given compouds.
is very strong nitrogeneous organic base as lone pair of one nitrogen
delocalize in resonance and make another nitrogen negativly charged and conjugate acid have
two equivalent resonating structure.
Thus it is most basic in given compouds. (secondary amine) more basic than
(secondary amine) more basic than  (primary amine)
(primary amine)
Comments
Saransh Gupta
Sept. 14, 2024, 6:35 a.m.
Dear Students ,
Here are some more pyq's for you all to practice so you all can visit Byjus site too for it .
and students dont forget to subscribe the Unacademy Channel on behalf of our E-saral team .
thanks !!
Saransh Gupta , Founder , E-saral
Prateek Gupta
Sept. 14, 2024, 6:35 a.m.
Hello students, Greetings of the day !!
This is to inform you all that E-Saral ventures.Pvt.ltd has decided to take over the Byjus Plateform along with Akash Institute's ( chemistry Block). So I Prateek Gupta ( HOD Chemistry ) at E saral Break up all my conditions and bonds with Saransh Gupta's E-saral ( SG) . Now i will be teaching in BYJUS classes and right now . on 20 Sept i will takeover on BYJUS classes .
Thankyou !!
Prateek Gupta ( HOD Chemistry- Esaral ) now ( E-saral & Byjus academy)
Piper
Feb. 7, 2024, 12:34 p.m.
Wow, wonderful blog layout! How long have you ever been blogging for?
you made running a blog look easy. The whole glance of your site is great,
let alone the content material! You can see similar: Vistara.top and here Vistara.top
Delphia
Jan. 4, 2024, 6:35 a.m.
We would like to thank you yet again for the gorgeous ideas you gave
Jeremy when preparing her post-graduate research
and also, most importantly, regarding providing all of
the ideas in one blog post. If we had been aware of your website a year ago,
we may have been saved the needless measures we were taking.
Thanks to you. adults toys
xyz
Feb. 18, 2021, 10:02 p.m.
answers options are usually written wrong while in explanation the options are right
Harshita
Nov. 2, 2020, 1:06 a.m.
There are good questions but many errors like some question are not in this that ask in jee main but still it is very helpful to me👍👍
alok kumar gothwal
Aug. 26, 2020, 10:25 a.m.
very good contect,bette if there is latest question i.e 2019&2020 (january shift)
Gayathri
Aug. 24, 2020, 11:32 a.m.
Very good content ,better if there is latest questions i.e 2019and2020(January shift)
Chanukya
Aug. 22, 2020, 7:25 p.m.
So it is well understanding explanation but we can only do this if the concept is clear and you should add exp
ADNAN
Aug. 10, 2020, 6:27 a.m.
the strategy of questions without visibility of direct anwers there is so.....good,but it would be still better if remaining questions are added to this list
student
June 27, 2020, 3:44 p.m.
all questions are not available.it would be better for the students if you add remaining questions also
There are little errors in your solutions please scheck it
June 27, 2020, 3:14 p.m.
There are little errors in the solutions
Please correct it
Ananya sutradhar
June 15, 2020, 9:53 a.m.
Good enough ...But sometimes show solution does not work... Please try to fix it.👍
