JEE Main Previous Year Papers Questions of Chemistry With Solutions are available at eSaral.
Simulator
Previous Years AIEEE/JEE Mains Questions
Q. Very pure hydrogen (99.9%) can be made by which of the following processes?
(1) Reaction of salt like hydrides with water
(2) Reaction of methane with steam
(3) Mixing natural hydrocarbons of high molecular weight
(4) Electrolysis of water
[AIEEE 2012]
Ans. (4)
Very pure hydrogen (99.9%) can be made by electrolysis of water.
Q. In which of the following reaction $\mathrm{H}_{2} \mathrm{O}_{2}$ acts as a reducing agent ?
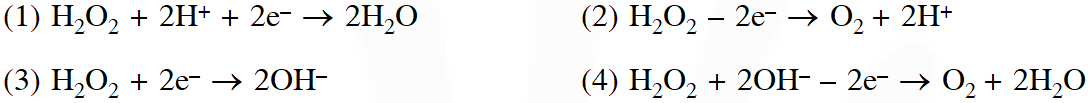 (1) (1), (3)
(2) (2), (4)
(3) (1), (2)
(4) (3), (4)
[JEE(Main) 2014]
(1) (1), (3)
(2) (2), (4)
(3) (1), (2)
(4) (3), (4)
[JEE(Main) 2014]
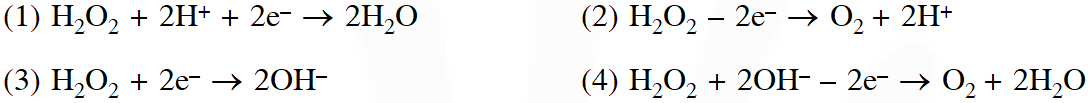 (1) (1), (3)
(2) (2), (4)
(3) (1), (2)
(4) (3), (4)
[JEE(Main) 2014]
(1) (1), (3)
(2) (2), (4)
(3) (1), (2)
(4) (3), (4)
[JEE(Main) 2014]
Ans. (2)
When $\mathrm{H}_{2} \mathrm{O}_{2}$ act as reducing agent then it evolve.
Q. Which of the following statements about $\mathrm{Na}_{2} \mathrm{O}_{2}$ is not correct ?
(1) $\mathrm{Na}_{2} \mathrm{O}_{2}$ oxidises $\mathrm{Cr}^{3+}$ to $\mathrm{CrO}_{4}^{2-}$ in acid medium
(2) It is diamagnetic in nature
(3) It is the super oxide of sodium
(4) It is a derivative of $\mathrm{H}_{2} \mathrm{O}_{2}$
[JEE(Main) 2014]
Ans. (3)
$\mathrm{Na}_{2} \mathrm{O}_{2}$ is peroxide of sodium
Q. Hydrogen peroxide acts both as an oxidising and as a reducing agent depending upon the nature of the reacting species. In which of the following cases $\mathrm{H}_{2} \mathrm{O}_{2}$ acts as a reducing agent in acid medium ? :-
(1) $\mathrm{MnO}_{4}^{-}$
(2) $\mathrm{SO}_{3}^{2-}$
(3) KI
(4) $\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}$
[JEE(Main)Online-2014]
Ans. (1)
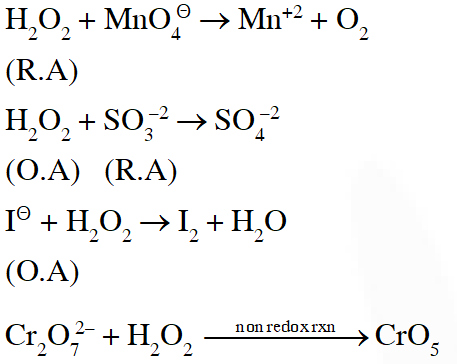
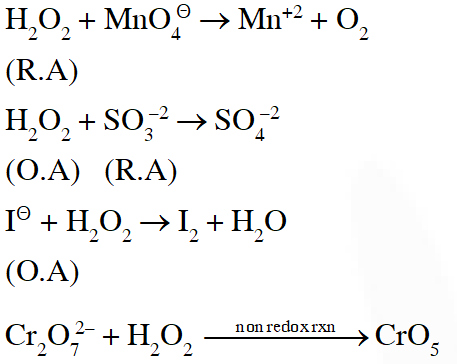
Q. Permanent hardness in water cannot be cured by:
(1) Treatment with washing soda
(2) Calgon's method
(3) Boiling
(4) Ion exchange method
[JEE(Main)Online-2015]
Ans. (3)
Permanent hardness in water cannot cured by boiling of water
Q. From the following statements regarding H2O2, choose the incorrect statement :
(1) It has to be stored in plastic or wax lined glass bottles in dark
(2) It has to be kept away from dust
(3) It can act only as an oxidizing agent
(4) It decomposes on exposure to light
[JEE(Main)Online-2015]
Ans. (3)
$\mathrm{H}_{2} \mathrm{O}_{2}$ can act as oxidizing as well as reducing agent depend on condition.
Q. Hydrogen peroxide oxidises $\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{4-}$ to $\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}$ in acidic medium but reduces
$\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}$ to $\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{4-}$ in alkaline
medium. The other products formed are, respectively :
[JEE(Main)Online-2018]
(1) $\left(\mathrm{H}_{2} \mathrm{O}+\mathrm{O}_{2}\right)$ and $\left(\mathrm{H}_{2} \mathrm{O}+\mathrm{OH}^{-}\right)$
(2) $\mathrm{H}_{2} \mathrm{O}$ and $\left(\mathrm{H}_{2} \mathrm{O}+\mathrm{O}_{2}\right)$
(3) $\mathrm{H}_{2} \mathrm{O}$ and $\left(\mathrm{H}_{2} \mathrm{O}+\mathrm{OH}^{-}\right)$
(4) $\left(\mathrm{H}_{2} \mathrm{O}+\mathrm{O}_{2}\right)$ and $\mathrm{H}_{2} \mathrm{O}$
Ans. (2)
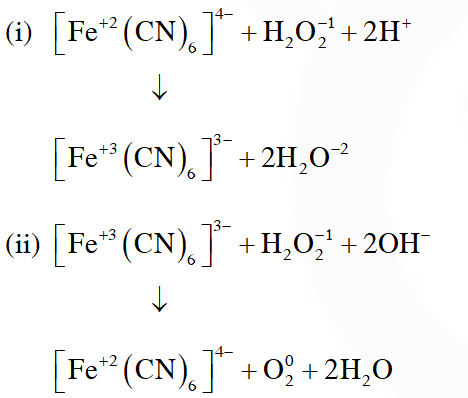
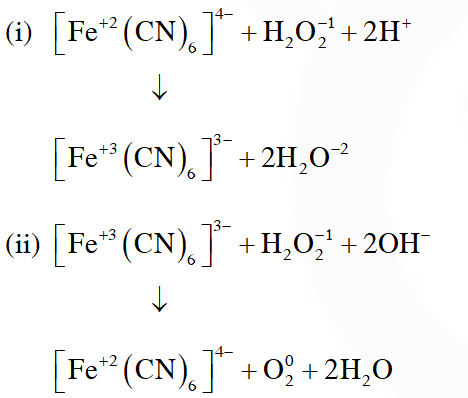
Comments
Father Chungus
Feb. 17, 2021, 10:56 p.m.
Very nice website big man, my children are little shits and they actually got some fecking education done here.
Small Chungus
Feb. 17, 2021, 10:53 p.m.
My brotha got says he got into iit NiggerNagar from cho website and I want in too, pls help man out.
Big Chungus
Feb. 17, 2021, 10:51 p.m.
Omaigawd so useful I did these questions, became tapir and then got into iit NiggerNagar.
Big Chungus
Feb. 17, 2021, 10:51 p.m.
Omaigawd so useful I did these questions, became tapir and then got into iit NiggerNagar
