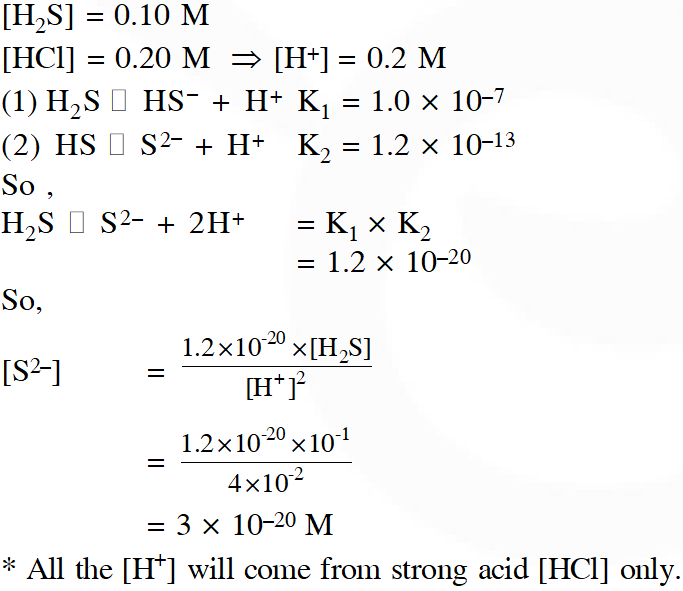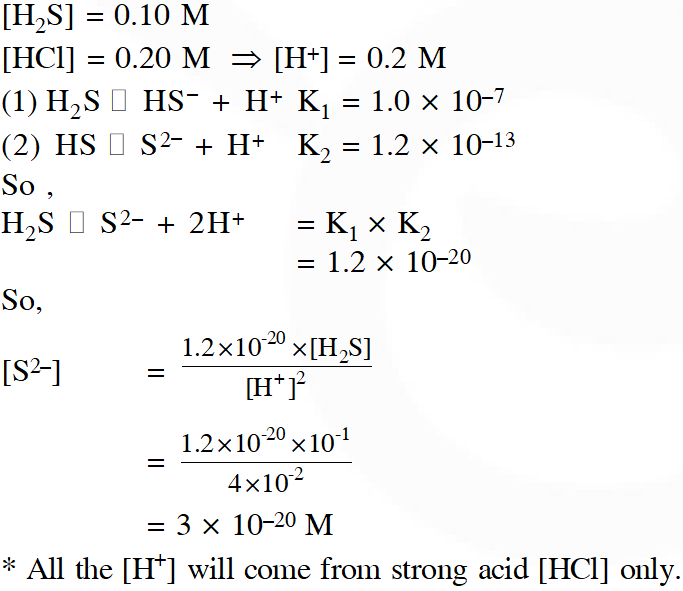JEE Main Previous Year Papers Questions of Chemistry With Solutions are available at eSaral.
Simulator
Previous Years AIEEE/JEE Mains Questions
Q. Solid Ba$\left(\mathrm{NO}_{3}\right)_{2}$ is gradully dissolved in a 1.0 × $10^{-4} \mathrm{M} \mathrm{Na}_{2} \mathrm{CO}_{3}$ solution.At what concentration of Ba2+ will a precipitate begin to form? $\left(\mathrm{K}_{\mathrm{SP}} \text { for } \mathrm{Ba} \mathrm{CO}_{3}=5.1 \times 10^{-9}\right)$
(A) $8.1 \times 10^{-8} \mathrm{M}$
(B) $8.1 \times 10^{-7} \mathrm{M}$
(C) $4.1 \times 10^{-5} \mathrm{M}$
(D) $5.1 \times 10^{-5} \mathrm{M}$
[AIEEE-2009,JEE-MAIN(Online)–2013]
Ans. (D)
$5.1 \times 10^{-9}=\left[\mathrm{Ba}^{+2}\right]\left[10^{-4}\right]$
$\left[\mathrm{Ba}^{+2}\right]=5.1 \times 10^{-5} \mathrm{M}$
Q. At 25° C, the solubility producct of $\mathrm{Mg}(\mathrm{OH})_{2}$ is $1.0 \times 10^{-11}$. At which pH, will $\mathrm{Mg}^{2+}$ ions start precipitating in the form of $\mathrm{Mg}(\mathrm{OH})_{2}$ from a solution of 0.001 M $\mathrm{Mg}^{2+}$ ions?
(A) 8 (B) 9 (C) 10 (D) 11
[AIEEE–2010]
Ans. (C)
$10^{-11}=\left[\mathrm{Mg}^{+2}\right]\left[\mathrm{OH}^{-}\right]^{2}$
$10^{-11}=\left(10^{-3}\right)\left[\mathrm{OH}^{-}\right]^{2}$
$\left[\mathrm{OH}^{-}\right]=10^{-4} \quad \mathrm{pOH}=4 \quad \mathrm{pH}=11$
Q. In aqueous solution the ionization constants for carbonic acid are $\mathrm{K}_{1}=4.2 \times 10^{-7}$ and $\mathrm{K}_{2}=4.8$ $\times 10^{-11}$ Select the correct statement for a saturated 0.034 M solution of the carbonic acid :-
(A) The concentration of $\mathrm{H}^{+}$ is double that of $\mathrm{CO}_{3}^{2-}$
(B) The concentration of $\mathrm{CO}_{3}^{2-}$ is $0.034 \mathrm{M}$
(C) The concentration of $\mathrm{CO}_{3}^{2-}$ is greater than that of $\mathrm{HCO}_{3}^{-}$
(D) The concentrations of $\mathrm{H}^{+}$ and $\mathrm{HCO}_{3}^{-}$ are approximately equal
[AIEEE–2010]
Ans. (D)
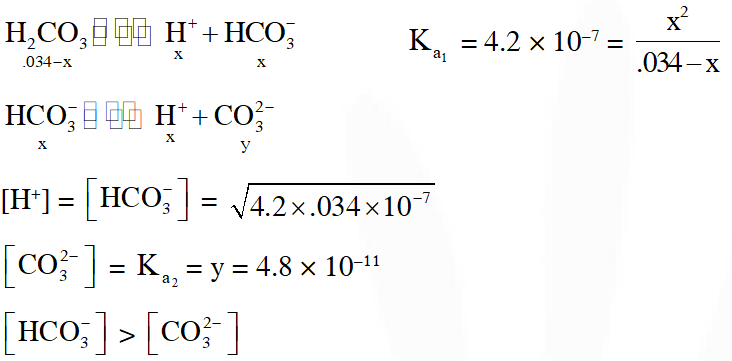
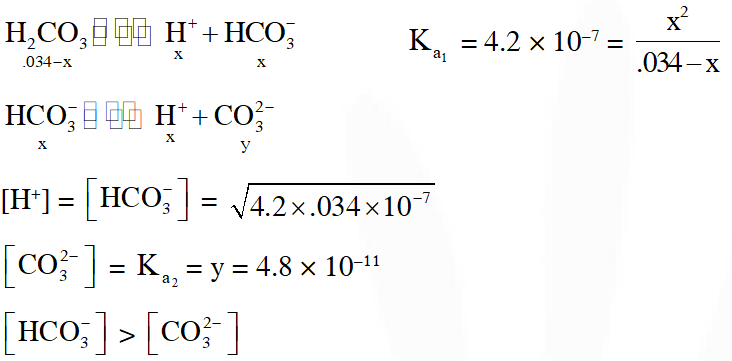
Q. Solubility product of silver bromide is $5.0 \times 10^{-13}$. The quantity of potassium bromide (molar mass taken as 120 g $\left.\mathrm{mol}^{-1}\right)$ to be added to 1 litre of 0.05 M solution of silver nitrate to start the precipitation of AgBr is :-
(A) $5.0 \times 10^{-8} \mathrm{g}$
(B) $1.2 \times 10^{-10} \mathrm{g}$
(C) $1.2 \times 10^{-9} \mathrm{g}$
(D) $6.2 \times 10^{-5} \mathrm{g}$
[AIEEE–2010]
Ans. (C)
$\left[\mathrm{Ag}^{+}\right]\left[\mathrm{Br}^{-}\right]=\mathrm{Ksp}$
$[0.05]\left[\frac{\mathrm{W}}{120}\right]=5 \times 10^{-13}$
$\mathrm{w}=\frac{120 \times 5 \times 10^{-13}}{5 \times 10^{-2}}=120 \times 10^{-11}=12 \times 10^{-10}$
$=1.2 \times 10^{-9} \mathrm{g}$
Q. An acid HA ionises as
 The pH of 1.0 M solution is 5. Its dissociation constant would be :-
(A) $1 \times 10^{-10}$
(B) 5
(C) $5 \times 10^{-8}$
(D) $1 \times 10^{-5}$
[AIEEE–2011]
The pH of 1.0 M solution is 5. Its dissociation constant would be :-
(A) $1 \times 10^{-10}$
(B) 5
(C) $5 \times 10^{-8}$
(D) $1 \times 10^{-5}$
[AIEEE–2011]
Ans. (A)
$\left[\mathrm{H}^{+}\right]=10^{-5}=\mathrm{C}_{\mathrm{o}} \alpha$
$\alpha=10^{-5}$
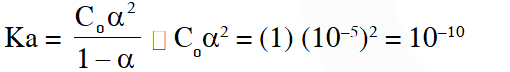
Q. The $\mathrm{K}_{\mathrm{sp}}$ for $\mathrm{Cr}(\mathrm{OH})_{3}$ is $1.6 \times 10^{-30}$ The molar solubility of this compound in water is :-
(A) $\sqrt[2]{1.6 \times 10^{-30}}$
(B) $\sqrt[4]{1.6 \times 10^{-30}}$
(C) $\sqrt[4]{1.6 \times 10^{-30} / 27}$
(D) $1.6 \times 10^{-30 / 27}$
[AIEEE–2011]
Ans. (C)
$\mathrm{Ksp}=1.6 \times 10^{-30}=27 \mathrm{S}^{4}$
$S^{4}=\left[\frac{1.6 \times 10^{-30}}{27}\right]$
$S=\left[\frac{1.6 \times 10^{-30}}{27}\right]^{1 / 4}$
Q. The pH of a 0.1 molar solution of the acid HQ is 3. The value of the ionization constant, Ka of this acid is :-
(A) $1 \times 10^{-7}$
(B) $3 \times 10^{-7}$
(C) $1 \times 10^{-3}$
(D) $1 \times 10^{-5}$
[AIEEE–2012]
Ans. (D)
[\mathrm{HQ}]=0.10 \mathrm{M}
\mathrm{pH}=3 \quad ;\left[\mathrm{H}^{+}\right]=10^{-3}=\mathrm{C}_{0} \alpha
\alpha=10^{-2}
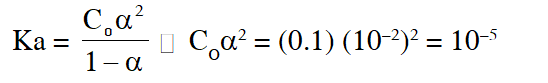
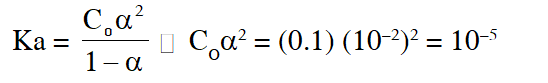
Q. If $\mathrm{K}_{\mathrm{sp}}$ of $\mathrm{CaF}_{2}$ at $25^{\circ} \mathrm{C}$ is $1.7 \times 10^{-10}$ , the combination amongst the following which gives a precipitate of $\mathrm{CaF}_{2}$ is :-
(A) $1 \times 10^{-2} \mathrm{M} \mathrm{Ca}^{2+}$ and $1 \times 10^{-5} \mathrm{M} \mathrm{F}^{-}$
(B) $1 \times 10^{-4} \mathrm{M} \mathrm{Ca}^{2+}$ and $1 \times 10^{-4} \mathrm{M} \mathrm{F}^{-}$
(C) $1 \times 10^{-3} \mathrm{M} \mathrm{Ca}^{2+}$ and $1 \times 10^{-5} \mathrm{M} \mathrm{F}^{-}$
(D) $1 \times 10^{-2} \mathrm{M} \mathrm{Ca}^{2+}$ and $1 \times 10^{-3} \mathrm{M} \mathrm{F}^{-}$
[JEE-MAIN(online)–2012]
Ans. (D)
Q. How many litres of water must be added to 1 litre of an aqueous solution of HCl with a pH of 1 to create an aqueous solution with pH of 2 ?
(A) 0.1 L (B) 0.9 L (C) 2.0 L (D) 9.0 L
[AIEEE–2013]
Ans. (D)
\left(10^{-1}\right)(1)=\left(10^{-2}\right)(1+\mathrm{v})
10=\mathrm{v}+1
v = 9L
Q. What would be the pH of a solution obtained by mixing 5 g of acetic acid and 7.5 g of sodium acetate and making the volume equal to 500 mL?
$\left(\mathrm{Ka}=1.75 \times 10^{-5}, \mathrm{pKa}=4.76\right)$
(A) 4.76 < pH < 5.0
(B) pH < 4.70
(C) pH of solution will be equal to pH of acetic acid
(D) pH = 4.70
[JEE-MAIN(Online)–2013]
Ans. (A)
Q. Which one of the following arrangements represents the correct order of solubilities of sparingly soluble salts $\mathrm{Hg}_{2} \mathrm{Cl}_{2}, \mathrm{Cr}_{2}\left(\mathrm{SO}_{4}\right)_{3}, \mathrm{BaSO}_{4}$ and $\mathrm{CrCl}_{3}$ respectively ?
(A) $\left(\frac{\mathrm{K}_{\mathrm{sp}}}{4}\right)^{\frac{1}{3}},\left(\frac{\mathrm{K}_{\mathrm{sp}}}{108}\right)^{\frac{1}{3}},\left(\mathrm{K}_{\mathrm{sp}}\right)^{\frac{1}{2}},\left(\frac{\mathrm{K}_{\mathrm{sp}}}{27}\right)^{\frac{1}{4}}$
(B) $\left(\mathrm{K}_{\mathrm{ap}}\right)^{\frac{1}{2}},\left(\frac{\mathrm{K}_{\mathrm{sp}}}{4}\right)^{\frac{1}{3}},\left(\frac{\mathrm{K}_{\mathrm{gp}}}{27}\right)^{\frac{1}{4}},\left(\frac{\mathrm{K}_{\mathrm{sp}}}{108}\right)^{\frac{1}{3}}$
(C) $\left(\mathrm{K}_{\mathrm{sp}}\right)^{\frac{1}{2}},\left(\frac{\mathrm{K}_{\mathrm{sp}}}{108}\right)^{\frac{1}{3}},\left(\frac{\mathrm{K}_{\mathrm{sp}}}{27}\right)^{\frac{1}{4}},\left(\frac{\mathrm{K}_{\mathrm{sp}}}{4}\right)^{\frac{1}{3}}$
$(\mathrm{D})\left(\frac{\mathrm{K}_{\mathrm{sp}}}{108}\right)^{\frac{1}{3}},\left(\frac{\mathrm{K}_{\mathrm{sp}}}{27}\right)^{\frac{1}{4}},\left(\mathrm{K}_{\mathrm{sp}}\right)^{\frac{1}{2}},\left(\frac{\mathrm{K}_{\mathrm{sp}}}{4}\right)^{\frac{1}{3}}$
[JEE-MAIN(Online)–2013]
Ans. (A)
Q. NaOH is a strong base. What will be pH of 5.0 × $10^{-2} \mathrm{M}$ NaOH solution ? (log2 = 0.3)
(A) 13.70 (B) 13.00 (C) 14.00 (D) 12.70
[JEE-MAIN(Online)–2013]
Ans. (D)
Q. Zirconium phosphate $\left[\mathrm{Zr}_{3}\left(\mathrm{PO}_{4}\right)_{4}\right]$ dissociates into three zirconium cations of charge +4 and four phosphate anions of charge –3. If molar solubility of zirconium phosphate is denoted by S and its solubility product by $\mathrm{K}_{\mathrm{sp}}$ then which of the following relationship between S and $\mathrm{K}_{\mathrm{sp}}$is correct ?
(A) $\mathrm{S}=\left\{\mathrm{K}_{\mathrm{sp}} / 144\right\}^{1 / 7}$
(B) $\mathrm{S}=\left\{\mathrm{K}_{\mathrm{sp}} /(6912)^{1 / 7}\right\}$
(C) $\mathrm{S}=\left(\mathrm{K}_{\mathrm{sp}} / 6912\right)^{1 / 7}$
(D) $\mathrm{S}=\left\{\mathrm{K}_{\mathrm{sp}} / 6912\right\}^{7}$
[JEE-MAIN(Online)–2014]
Ans. (C)
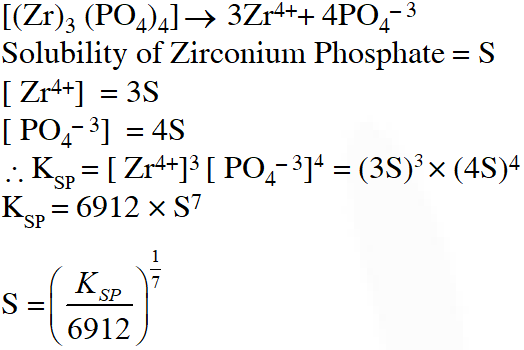
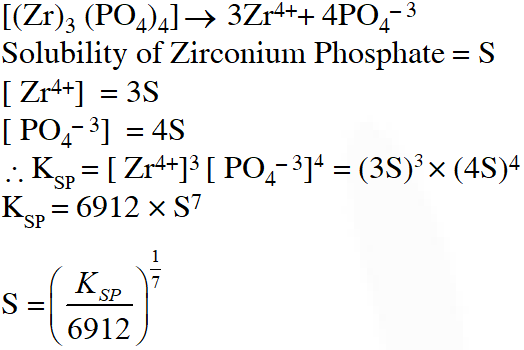
Q. In some solutions, the concentration of $\mathrm{H}_{3} \mathrm{O}^{+}$ remains constant even when small amounts of strong acid or strong base are added to them. These solutions are known as :-
(A) Colloidal solutions (B) True solutions
(C) Ideal solutions (D) Buffer solutions
[JEE-MAIN(Online)–2014]
Ans. (D)
Q. An aqueous solution contains 0.10 M $\mathrm{H}_{2} \mathrm{S}$ and 0.20 M HCl. If the equilibrium constants for the formation of HS– from H2S is 1.0 × $10^{-7}$ and that of $\mathrm{S}^{2-}$ from $\mathrm{HS}^{-}$ ions is 1.2×$10^{-13}$ then the concentration of $\mathrm{S}^{2-}$ ions in aqueous solution is :
(A) $3 \times 10^{-20}$
(B) $6 \times 10^{-21}$
(C) $5 \times 10^{-19}$
(D) $5 \times 10^{-8}$
[JEE-MAIN–2018]
Ans. (A)