Metals and Non-Metals Class 10 Notes
Class 10
In Metals and Non-Metals Class 10 Notes , we will explore the fascinating world of Metals and Non-Metals. We will learn about the physical and chemical properties of these two categories of elements and how they differ from each other. We will delve into their atomic structures, and their reactions with each other and with other elements. We will also study the uses and applications of metals and non-metals in various industries and in our everyday lives. Metals and Non-Metals Class 10 Notes are important for Class 10 students as it forms the basis for further studies in Chemistry and Material Science. By the end of this chapter, you will have a comprehensive understanding of the properties and characteristics of metals and non-metals.
Metals conduct heat and electricity and are malleable and ductile. Examples include Iron (Fe), Aluminium (Al), Silver (Ag), Copper (Cu), Gold (Au), Platinum (Pt), Lead (Pb), Potassium (K), Sodium (Na), Calcium (Ca), and Magnesium (Mg).
Metals lose electrons to form positive ions, so they are Electropositive Elements.
To get Class 10 Complete video lectures taught by IITian and Doctor faculties, Complete study material, test series and much more, Download the eSaral App.
Physical Properties of Metals
-
Metals, with the exception of alkali metals like sodium, potassium, lithium, etc., are typically hard and cannot be cut with a knife.
-
Most metals have high tensile strength, which makes them ideal for constructing large structures such as copper (Cu) and iron (Fe). However, soft metals such as sodium (Na) and potassium (K) are not as strong.
-
Metals are typically solid at room temperature, except for mercury (Hg).
-
Metals produce a ringing sound, which is why they are called Sonorous. This sound is also known as Metallic sound, and metal wires are often used in musical instruments.
-
Metals are excellent conductors of heat and electricity, which is why electric wires are made of metals like copper and aluminium.
-
Metals are malleable, meaning they can be beaten into thin sheets. This property is why iron is commonly used in shipbuilding.
-
Metals are ductile, meaning they can be drawn into thin wires. This property is why most wires are made of metals.
-
Metals generally have high melting and boiling points, although soft metals like sodium and potassium have low melting and boiling points.
-
Most metals have a high density.
-
While most metals are grey in colour, gold and copper are exceptions.
Chemical Properties of Metals
Reaction with oxygen: Most of the metals form respective metal oxides when reacting with oxygen.
Metal + Oxygen → Metal Oxide
Examples:
-
Magnesium + Oxygen → Magnesium Oxide
2Mg + O2 → 2MgO -
Aluminium + Oxygen → Aluminium Oxide
4Al + 3O2 → 2Al2O3
Copper metal reacts with oxygen in the air to form copper oxide. Here is the equation for the reaction:
2Cu + O2 → 2CuO
In this reaction, two atoms of copper (Cu) combine with one molecule of oxygen gas (O2) to form two molecules of copper oxide (CuO). This is an example of a redox reaction, where copper is oxidized (loses electrons) and oxygen is reduced (gains electrons). The resulting copper oxide is a black solid that forms on the surface of the copper metal. This reaction is also known as the process of copper oxidation, which is responsible for the green patina that forms on copper over time due to the reaction of copper metal with air and moisture.
Reaction of metals with water: Metals form respective hydroxide and hydrogen gas when reacting with water.
When metals react with water, they can form metal hydroxides and hydrogen gas. The reactivity of the metal with water determines the speed and intensity of the reaction. Here are some examples of metal-water reactions:
-
Sodium + Water → Sodium Hydroxide + Hydrogen Gas 2Na + 2H2O → 2NaOH + H2
-
Calcium + Water → Calcium Hydroxide + Hydrogen Gas Ca + 2H2O → Ca(OH)2 + H2
-
Potassium + Water → Potassium Hydroxide + Hydrogen Gas 2K + 2H2O → 2KOH + H2
-
Magnesium + Water → Magnesium Hydroxide + Hydrogen Gas Mg + 2H2O → Mg(OH)2 + H2
-
Zinc + Water → Zinc Hydroxide + Hydrogen Gas Zn + 2H2O → Zn(OH)2 + H2
In these reactions, the metal (sodium, calcium, potassium, magnesium, or zinc) reacts with water to form the corresponding metal hydroxide (sodium hydroxide, calcium hydroxide, potassium hydroxide, magnesium hydroxide, or zinc hydroxide) and hydrogen gas (H2). The metal hydroxide is a base that dissolves in water to form hydroxide ions (OH-) and the corresponding metal cations (Na+, Ca2+, K+, Mg2+, or Zn2+). The hydrogen gas is produced when water molecules are split into hydrogen ions (H+) and hydroxide ions (OH-) by the metal.
Reaction of metals with dilute acid: Metals form respective salts when reacting with dilute acid.
Metal + dil. acid → Metal salt + Hydrogen
When metals react with dilute acid, they can form metal salts and hydrogen gas. The reactivity of the metal with acid determines the speed and intensity of the reaction. Here are some examples of metal-acid reactions:
-
Zinc + Dilute Hydrochloric Acid → Zinc Chloride + Hydrogen Gas Zn + 2HCl → ZnCl2 + H2
-
Iron + Dilute Sulfuric Acid → Iron Sulfate + Hydrogen Gas Fe + H2SO4 → FeSO4 + H2
-
Magnesium + Dilute Hydrochloric Acid → Magnesium Chloride + Hydrogen Gas Mg + 2HCl → MgCl2 + H2
-
Copper + Dilute Nitric Acid → Copper Nitrate + Nitrogen Dioxide Gas + Water Cu + 4HNO3 → Cu(NO3)2 + 2NO2 + 2H2O
-
Aluminium + Dilute Sulfuric Acid → Aluminium Sulfate + Hydrogen Gas 2Al + 3H2SO4 → Al2(SO4)3 + 3H2
In these reactions, the metal (zinc, iron, magnesium, copper, or aluminium) reacts with the dilute acid (hydrochloric acid, sulfuric acid, or nitric acid) to form the corresponding metal salt (zinc chloride, iron sulfate, magnesium chloride, copper nitrate, or aluminium sulfate) and hydrogen gas (H2). In the case of copper and nitric acid, nitrogen dioxide gas (NO2) and water (H2O) are also produced. The metal salt is an ionic compound that dissolves in water to form metal cations (Zn2+, Fe2+, Mg2+, Cu2+, or Al3+) and anions (Cl-, SO42-, NO3-, or OH-). The hydrogen gas is produced when the acid molecules donate hydrogen ions (H+) to the metal, which reduces the hydrogen ions to hydrogen gas (H2).
Metal Oxides
Chemical Properties: Metal oxides are basic in nature. The aqueous solution of metal oxides turns red litmus blue.
Metal oxides can react with water to form metal hydroxides, which can be either acidic, basic, or neutral depending on the nature of the oxide. Here are some examples of metal oxide-water reactions:
-
Calcium Oxide + Water → Calcium Hydroxide CaO + H2O → Ca(OH)2
-
Magnesium Oxide + Water → Magnesium Hydroxide MgO + H2O → Mg(OH)2
-
Sodium Oxide + Water → Sodium Hydroxide Na2O + H2O → 2NaOH
-
Potassium Oxide + Water → Potassium Hydroxide K2O + H2O → 2KOH
In these reactions, metal oxides (calcium oxide, magnesium oxide, sodium oxide, or potassium oxide) react with water to form the corresponding metal hydroxides (calcium hydroxide, magnesium hydroxide, sodium hydroxide, or potassium hydroxide). Calcium oxide and magnesium oxide are basic oxides, which react with water to form basic metal hydroxides. Sodium oxide and potassium oxide are alkali metal oxides, which react with water to form strong alkali metal hydroxides. These alkali metal hydroxides are strong bases, which can react with acids to form salts and water.
Reactivity Series of Metals:
Metals and Non-Metals Class 10 Notes-The Reactivity Series is a sequence that indicates the order of reactivity of metals. Moving from top to bottom in the Reactivity Series, the reactivity of elements decreases. At the bottom of the series, copper, gold, and silver are located, which makes them the least reactive metals. These metals are referred to as Noble metals. Conversely, at the top of the series, potassium is located and thus, is the most reactive metal.
| Metal | Reactivity |
|---|---|
| Potassium | Most reactive |
| Sodium | |
| Calcium | |
| Magnesium | |
| Aluminium | |
| Zinc | |
| Iron | |
| Nickel | |
| Tin | |
| Lead | |
| Hydrogen | |
| Copper | Least reactive |
| Mercury | |
| Silver | |
| Gold | Noble metal |
| Platinum | |
| Palladium |
Note: The metals at the top of the table are the most reactive, and their reactivity decreases as you move down the table. The Noble metals such as gold, silver, and platinum are the least reactive. The Reactivity Series helps predict the likelihood of a metal reacting with other substances, such as acids.
Reaction of metals with solution of other metal salts
The reaction of metals with the solution of other metal salt is called a displacement reaction. In this reaction, the more reactive metal displaces the less reactive metal from its salt, and a new salt and a new metal are formed. Here are some examples and equations of displacement reactions:
-
Zinc + Copper(II) sulfate → Zinc sulfate + Copper Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
-
Iron + Copper(II) sulfate → Iron(II) sulfate + Copper Fe(s) + CuSO4(aq) → FeSO4(aq) + Cu(s)
-
Magnesium + Iron(II) chloride → Magnesium chloride + Iron Mg(s) + FeCl2(aq) → MgCl2(aq) + Fe(s)
In the above examples, zinc, iron, and magnesium are more reactive than copper and iron, respectively. Therefore, they displace copper and iron from their salts, and new salts and metals are formed.
Non-Metals:
Metals and Non-Metals Class 10 Notes-Non-metals are elements that lack the ability to conduct electricity and are not malleable or ductile. Examples of non-metals include carbon (C), sulfur (S), phosphorus (P), silicon (Si), hydrogen (H), oxygen (O), nitrogen (N), chlorine (Cl), bromine (Br), neon (Ne), and argon (Ar).
Non-metals form negative ions by gaining electrons, which makes them electronegative elements.
Non-metal oxides are formed when non-metals react with oxygen. These oxides are acidic in nature and can react with bases to form salts and water.
When metals react with non-metals, they form ionic bonds. Ionic bonds are formed when one element loses an electron to form a positively charged ion (cation), while another element gains an electron to form a negatively charged ion (anion). The attraction between the cation and anion forms an ionic bond.
The chemical properties of non-metals differ from those of metals. Non-metals have a higher electronegativity, which means they tend to gain electrons and form negative ions. They also have lower melting and boiling points than metals.
In summary, non-metals have physical and chemical properties that distinguish them from metals, including their inability to conduct electricity and lack of malleability or ductility. They form negative ions and non-metal oxides, and can participate in the formation of ionic bonds.
Physical Properties of non-metals
- Hardness: Non-metals are not hard rather they are generally soft. But the diamond is an exception; it is the hardest naturally occurring substance.
- State: Non-metals may be solid, liquid or gas.
- Lustre: Non-metals have a dull appearance. Diamond and iodine are exceptions.
- Sonority: Non-metals are not sonorous, i.e., they do not produce a typical sound on being hit.
- Conduction: Non-metals are a bad conductor of heat and electricity. Graphite which is allotrope of carbon is a good conductor of electricity and is an exception.
- Malleability and ductility: Non-metals are brittle.
- Melting and boiling point: Non-metals have generally low melting and boiling points.
- Density: Most of the non-metals have low density.
- Colour: Non-metals are in many colours.
Carbon in the form of graphite is non-metal which conduct electricity.
Iodine is non-metal which is lustrous having a shining surface.
Carbon in the form of diamond is a non-metal which is extremely hard.
Diamond is a non-metal which has a very high melting point and boiling point
Chemical properties of Non-metals
Reaction of Non-metals with Oxygen: Non-metals form respective oxide when reacting with oxygen.
Non-metal + Oxygen → Non-metallic oxide
-
Carbon + Oxygen → Carbon dioxide
C + O2 → CO2 -
Sulphur + Oxygen → Sulphur dioxide
S + O2 → SO2 -
Phosphorus + Oxygen → Phosphorus pentoxide
4P + 5O2 → P4O10 -
Nitrogen + Oxygen → Nitrogen dioxide
2N2 + O2 → 2NO2 -
Chlorine + Oxygen → Chlorine dioxide
2Cl + 3O2 → 2ClO2
Non-metallic Oxide
Non-metallic oxides are acidic in nature. The solution of non-metal oxides turns blue litmus red.
-
Sulphur dioxide + Water → Sulphurous acid SO2 + H2O → H2SO3
-
Nitrogen dioxide + Water → Nitric acid NO2 + H2O → HNO3
-
Carbon dioxide + Water → Carbonic acid CO2 + H2O → H2CO3
-
Phosphorus pentoxide + Water → Phosphoric acid P4O10 + 6H2O → 4H3PO4
In each of these reactions, the non-metal oxide dissolves in water to form an acidic solution. The acidic solution turns blue litmus paper red.
Reaction of Non-metal with Chlorine
Non-metal gives respective chloride when they react with chlorine gas.
Non-metal + Chlorine → Non-metal chloride
Hydrogen gives hydrogen chloride and phosphorous gives phosphorous trichloride when reacting with chlorine.
When non-metals react with chlorine gas, they give their respective chlorides.
For example, hydrogen reacts with chlorine gas to give hydrogen chloride:
H2 + Cl2 → 2HCl
Similarly, phosphorous reacts with chlorine gas to give phosphorous trichloride:
P4 + 6Cl2 → 4PCl3
Reaction of Non-metals with Hydrogen:
Hydrogen sulphide is formed when sulphur reacts with hydrogen gas. The equation for the reaction is:
Sulfur + Hydrogen → Hydrogen sulfide S + H2 → H2S
Similarly, another example of a non-metal reacting with hydrogen to form a covalent hydride is:
Nitrogen + Hydrogen → Ammonia N2 + 3H2 → 2NH3
Reaction of Metal and Non-metal:
When metals react with non-metals, they tend to lose electrons and form positive ions. These positive ions are attracted to the negative ions of non-metals, resulting in the formation of ionic compounds.
Examples:
- Sodium (Na) reacts with chlorine (Cl) to form sodium chloride
(NaCl) Na + Cl → NaCl
In this reaction, sodium loses one electron to form a positively charged ion, Na+. Chlorine gains one electron to form a negatively charged ion, Cl-. The attraction between Na+ and Cl- results in the formation of the ionic compound NaCl.
- Calcium (Ca) reacts with oxygen (O) to form calcium oxide
(CaO) 2Ca + O2 → 2CaO
In this reaction, each calcium atom loses two electrons to form a Ca2+ ion, while each oxygen atom gains two electrons to form an O2- ion. The resulting ionic compound is CaO.
Positive ions are formed when metals lose electrons, resulting in a decrease in their electron cloud size and a corresponding increase in positive charge
A negative ion is formed when an atom gains an electron. Some examples are given below: Chlorine gains one electron in order to achieve a stable configuration. After gaining one electron, chlorine gets one negative charge over it forming chloride ion.
Cl + e- → Cl-
Note: The equation shows the process of chlorine gaining one electron to become a chloride ion.
Ionic Bonds
The formation of Ionic bonds occurs through the transfer of electrons from a metal to a non-metal, resulting in the metal acquiring a positive charge while the non-metal becomes negatively charged. The resulting bond between the positively and negatively charged ions is known as an Ionic Bond.
To form an ionic compound, negative and positive ions must combine in order to maintain the electrical neutrality of the compound.
Some examples are given below:
Formation of Sodium Chloride (NaCl): In sodium chloride, sodium is a metal (alkali metal) and chlorine is a non-metal.
Atomic number of sodium = 11
Electronic configuration of sodium : 2, 8, 1
Number of electrons in outermost orbit = 1
Valence electrons = Electrons in outermost orbit = 1
Atomic number of chlorine = 17
Electronic configuration of chlorine : 2, 8, 7
Electrons in outermost orbit = 7
Therefore, valence electrons = ?
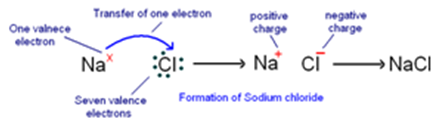
Sodium and chlorine have different valence electrons: sodium has one and chlorine has seven. To achieve stable electronic configurations, sodium must lose one electron, and chlorine must gain one electron. Therefore, sodium transfers one electron to chlorine to obtain a stable electronic configuration. As a result, sodium becomes positively charged (+1), and chlorine becomes negatively charged (-1) due to the gained electron. The transfer of electrons between them forms an ionic bond. The compound formed by this ionic bond is called sodium chloride (NaCl) or table salt. Similarly, potassium chloride (KCl) is formed in a similar manner.
Properties of Ionic compound
Metals and Non-Metals Class 10 Notes-The force of attraction between ions in an ionic bond is greater, which results in the solid nature of ionic compounds. Additionally, ionic compounds are brittle due to the rigid arrangement of ions in their crystal lattice structure.
The high melting and boiling points of ionic compounds are attributed to the strong force of attraction between their ions. Ionic compounds typically dissolve in water due to the polarity of water molecules, which helps in separating and surrounding the ions.
In contrast, organic solvents such as kerosene and petrol are generally non-polar and cannot dissolve ionic compounds. Ionic compounds in the solid state do not conduct electricity because their ions are held in fixed positions. However, the solution of ionic compounds in water can conduct electricity due to the mobility of ions in solution.
Finally, ionic compounds can also conduct electricity in the molten state, as the ions are free to move around in the absence of the rigid crystal lattice structure present in the solid state.
Occurrence and Extraction of Metals:
Metals are sourced from Earth's crust and seawater, primarily in the form of ores. Minerals, which have a uniform composition, are naturally occurring substances. Ores are minerals from which metals can be extracted profitably.
Metals at the bottom of the reactivity series, such as gold, silver, and copper, are the least reactive and are often found in nature in a free state. Copper and silver are also found as sulphide and oxide ores.
Metals in the middle of the reactivity series, such as Zn, Fe, and Pb, are usually found as oxides, sulphides, or carbonates.
Metals at the top of the reactivity series, such as K, Na, Ca, Mg, and Al, are very reactive and are never found in free-state. Many metals are found in the form of oxides as oxygen is abundant in nature and highly reactive.
The extraction of metals involves three major steps based on their reactivity: most reactive, medium reactive, and least reactive. These steps include concentration or enrichment of ores, conversion of concentrated ore into crude metal, and refining of impure or crude metal.
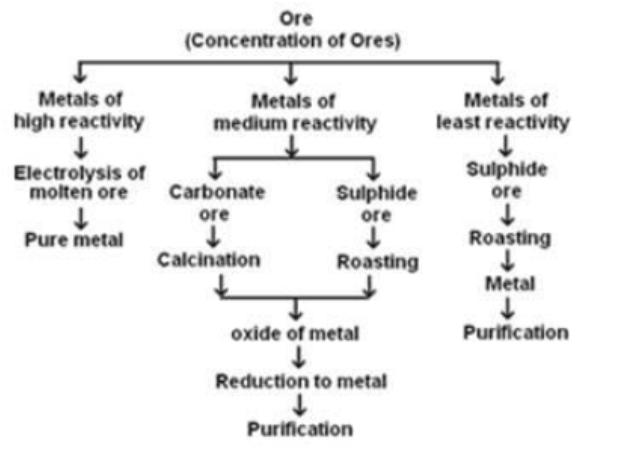
Concentration of Ores:
- Concentration of ores involves the removal of impurities from mined ores.
- Impurities in ores are called gangue.
- Concentration is the first step in the extraction of metals from ores.
- The process of concentration is also called enrichment of ores.
- The concentration process is dependent on the physical and chemical properties of the ores.
- Several processes such as gravity separation, electromagnetic separation, and froth flotation process can be used for concentration of ores.
Conversion of Concentrated Ore into Crude Metal
It is easy to obtain metals from their oxides. So, ores found in the form of sulphide and carbonates are first converted to their oxides by the process of roasting and calcination. Oxides of metals so obtained are converted into metals by the process of reduction.
Roasting is a process in which sulphide ores are heated in the presence of excess air to convert them into oxides. This process can be represented by the following equation:
2MS (s) + 3O2 (g) → 2MO (s) + 2SO2 (g)
Where MS represents the sulphide ore and MO represents the oxide formed.
Calcination is the process of heating the carbonate ores in a limited supply of air to convert them into metal oxides, while driving off carbon dioxide gas. The general equation for calcination of a carbonate ore is:
Metal carbonate → Metal oxide + Carbon dioxide gas
For example, calcination of calcium carbonate (limestone) results in the formation of calcium oxide (quicklime) and carbon dioxide gas as shown below:
CaCO3 (s) → CaO (s) + CO2 (g)
Difference between Roasting and calcination
| Calcination | Roasting |
|---|---|
| Heating of carbonate ores in limited air | Heating of sulphide ores in excess air |
| The process is done to convert carbonate ores into oxides | The process is done to convert sulphide ores into oxides |
| Carbonate ores are decomposed by heat to form oxides and carbon dioxide | Sulphide ores are converted into oxides and sulphur dioxide |
| The reaction is endothermic | The reaction is exothermic |
| Examples: heating of limestone to form lime | Examples: heating of zinc blende to form zinc oxide |
| Used in the extraction of metals such as calcium, magnesium, and zinc | Used in the extraction of metals such as copper, lead, and iron |
Overall, both calcination and roasting are important processes in the extraction of metals, and they are distinguished by the types of ores that they are used on and the conditions under which they are carried out.
Reduction is Heating of oxides of metals to turn them into metal.
Extraction of Metals of Least Reactivity - Metals and Non-Metals Class 10 Notes
Mercury and copper, which belong to the least reactivity series, are often found in the form of their sulphide ores. The process of extracting mercury metal from cinnabar (HgS) and copper metal from copper glance (Cu2S) is given below:
Extraction of Mercury Metal:
- Cinnabar (HgS) is heated in air to obtain HgO and SO2: HgS + O2 → HgO + SO2
- Mercury oxide obtained is heated strongly to obtain mercury metal: 2HgO → 2Hg + O2
Extraction of Copper Metal:
- Copper glance (Cu2S) is roasted in the presence of air to form copper oxide (CuO) and sulphur dioxide (SO2): 2Cu2S + 3O2 → 2CuO + 2SO2
- Copper oxide is reduced to copper metal by heating with coke (carbon) in a blast furnace: CuO + C → Cu + CO2
Extraction of Copper Metal -Metals and Non-Metals Class 10 Notes
Metals and Non-Metals Class 10 Notes-To extract copper metal, we first roast copper glance (Cu2S) in the presence of air. The chemical equation for this reaction is:
2Cu2S + 3O2 → 2Cu2O + 2SO2
The copper glance (Cu2S) is converted into copper (I) oxide (Cu2O) and sulphur dioxide (SO2) is liberated.
Next, copper (I) oxide is heated in the absence of air. This reduces copper (I) oxide into copper metal. The chemical equation for this reaction is:
2Cu2O → 4Cu + O2
So, copper (I) oxide is reduced to copper metal and oxygen gas is liberated.
Extraction of Metals of Middle Reactivity:
Metals and Non-Metals Class 10 Notes-Iron, zinc, lead, etc. are found in the form of carbonate or sulphide ores. Carbonate or sulphide ores of metals are first converted into respective oxides and then oxides are reduced to respective metals.
Extraction of Zinc: Roasting of Zinc Blende: Zinc blende (ZnS), which is the ore of zinc, is roasted in the presence of air. This converts ZnS into ZnO and SO2 is liberated.
ZnS + 2O2 → ZnO + 2SO2
Calcination of Zinc Spar: Zinc spar (ZnCO3), another ore of zinc, is put under calcination in a limited supply of air. This converts ZnCO3 into ZnO and CO2 is liberated.
ZnCO3 → ZnO + CO2
Extraction of Iron from Haematite : Heating of haematite (Fe2O3) ore in the presence of carbon (C) is known as reduction of haematite ore. This reaction results in the production of iron metal (Fe) and carbon dioxide (CO2). The balanced chemical equation for the reaction is:
2Fe2O3 + 3C → 4Fe + 3CO2
Extraction of Lead from Lead oxide: Lead oxide (PbO) is heated with carbon (C) to reduce it to lead metal (Pb) as shown below:
PbO + C → Pb + CO
Reduction of Metal oxide by Heating with Aluminium: When aluminium is heated with metal oxides, it acts as a reducing agent and reduces the metal oxides to their respective metals. This can be demonstrated by the following equations for the reduction of manganese dioxide and copper oxide to their respective metals:
Manganese dioxide + 2Al → Manganese + Aluminium oxide + Heat (3MnO2 + 4Al → 3Mn + 2Al2O3 + Heat)
Copper oxide + 2Al → Copper + Aluminium oxide + Heat (2CuO + 2Al → 2Cu + Al2O3 + Heat)
Thermite Reaction: When aluminium is heated with ferric oxide, it reduces to iron metal, and a significant amount of heat is generated. This process is called the thermite reaction, which is utilized in welding electric conductors and iron joints, including railway track joints. This reaction is also known as Thermite Welding (TW).
The chemical equation for the reaction can be written as:
Fe2O3 + 2Al → 2Fe + Al2O3 + Heat
The process of extracting metals of high reactivity, such as sodium, calcium, magnesium, aluminium, etc. from their ores involves electrolytic reduction. Carbon cannot be used to reduce these metals since carbon is less reactive than them.
Electrolytic Reduction: In electrolytic reduction, the molten state of metal ores is subjected to an electric current, which causes the positively charged metal ions to be deposited onto the cathode. For example, when an electric current is passed through molten sodium chloride, sodium metal is deposited onto the cathode. This process can be represented by the following equation:
2NaCl (molten) → 2Na+ + 2Cl- 2Na+ + 2e- → 2Na (deposition at the cathode)
Refining or purification of metals
Metals and Non-Metals Class 10 Notes -Metals extracted from various methods contains some impurities, thus, they are required to be refined. Most of the metals are refined using electrolytic refining.
Electrolytic Refining: The process of electrolytic refining involves immersing a lump of impure metal and a thin strip of pure metal in a salt solution of the same metal. Passing an electric current through the solution results in the deposition of pure metal over the strip of pure metal.
Electrolytic Refining of Copper: When performing electrolytic refining of copper, a lump of impure copper metal and a thin strip of pure copper are placed in a solution of copper sulphate. The impure lump of metal is connected to the positive pole and the thin strip of pure metal is connected to the negative pole. Passing an electric current through the solution causes pure copper from the anode to move towards the cathode and be deposited over it. The impurities present in the metal settle near the bottom of the anode in the solution, forming what is called anode mud. The process can be represented by the following equations:
At the anode: Cu(s) → Cu2+(aq) + 2e−
At the cathode: Cu2+(aq) + 2e− → Cu(s)
Overall: Cu(s) + Cu2+(aq) → 2Cu(s)
Corrosion:
The atmospheric air reacts with most metals, leading to the formation of a layer over the metal. Over time, the underlying layer of metal gets lost due to conversion into oxides, sulphides, carbonates, or other compounds. As a result, the metal corrodes or deteriorates. This process is called corrosion.
Rusting of Iron: The most common form of corrosion is the rusting of iron. When iron articles such as gates, grills, fences, etc. come into contact with moisture in the air, the upper layer of iron reacts with oxygen to form iron oxide, which is brown-red in colour and known as rust. This phenomenon is called rusting of iron. If rusting is not prevented in time, the entire iron article will continue to oxidize and turn into iron oxide. This process is also known as corrosion of iron. Rusting of iron causes significant losses every year if not prevented in time.
Prevention of Rusting: For rusting, iron must come in contact with oxygen and water. Rusting is prevented by preventing the reaction between atmospheric moisture and the iron article. This can be done by:
- Painting
- Greasing
- Galvanization
- Electroplating
- Alloying
Alloys
he homogeneous mixture of two or more metals, or a metal and a non-metal is called Alloy.
Types of alloys :
- Ferrous alloys: An alloy in which iron (Fe) is present. For example : manganese steel (Fe = 86% ; Mn = 13% ; C = 1%) and Nickle steel (Fe = 98% ; Ni = 2%).
- Non-ferrous alloys: An alloy does not contain iron. For example : Brass (Cu = 80% ; Zn = 20%), and Bronze (Cu = 90% ; Sn = 10%).
- Amalgams: An alloy in which mercury (Hg) is present. For example Sodium amalgams [Na(Hg)] and Zinc amalgams [Zn(Hg)].
Properties of an Alloy
- Alloys are stronger than the metal from which they are obtained.
- It is harder than the constituent metals.
- More resistance to corrosion.
- The melting point of alloys is lower than the constituent metals.
Example: Solder [Sn(80%) + Pb(50%)] has lower m. p. than Pb and Sn. - The electrical conductivity of alloys is lower than the constituent metals.
Some examples of Alloys:
- Brass: [80% Cu + 20% Zn ]
- Bronze: [90% Cu + 20% Sn]
- Solder: [50% Pb + 50% Sn]
- Duralumin: [95% Al + 4% Cu + 0.5% Mg + 0.5 Mn]
- Steel: [99.95% Fe + 0.05% C]
- Stainless steel: [74% Fe + 18% Cr + 8% Ni]
- Magnesium: [95% Al + 5% Mg]
- German Silver: [60% Cu + 20% Zn + 20% Ni]
- Alloys of Gold: Pure gold is said to be of 24 carats. Gold is alloyed with a small amount of silver or copper to make it hard.
Metals and Non-metals
| Metals | Nonmetals |
|---|---|
| Elements that are typically hard, shiny, malleable, ductile, and good conductors of heat and electricity | Elements that are typically brittle, dull, and poor conductors of heat and electricity |
| Found on the left side and center of the periodic table | Found on the right side of the periodic table |
| Tend to lose electrons and form positively charged ions (cations) | Tend to gain electrons and form negatively charged ions (anions) |
| Most are solids at room temperature, except for mercury (Hg) which is a liquid | Can be solids, liquids, or gases at room temperature |
| Have low electronegativity and low ionization energies | Have high electronegativity and high ionization energies |
| Form basic oxides when they react with oxygen | Form acidic or neutral oxides when they react with oxygen |
| Typically have high melting and boiling points | Typically have low melting and boiling points |
| React with acids to form salt and hydrogen gas | Do not react with acids in the same way as metals |
Chemical Properties of Metals and Non-metals.- Metals and Non-Metals Class 10 Notes
Metals and Non-metals Reactivity with Oxygen, Water, Acid, and Base:
Reaction with Oxygen:
Metal: Metal + Oxygen → Metal oxide (basic in nature) Example: Iron + Oxygen → Iron oxide
Non-metal: Non-metal + Oxygen → Non-metal oxide (acidic in nature) Example: Sulphur + Oxygen → Sulphur dioxide
Reaction with Water:
Metal: Metal + Water → Metal hydroxide Example: Sodium + Water → Sodium hydroxide + Hydrogen
Non-metal: Generally do not react with water, but some stored in water. Example: N/A
Reaction with Dilute Acid:
Metal: Metal + Acid → Hydrogen gas + Salt Example: Zinc + Hydrochloric acid → Zinc chloride + Hydrogen
Non-metal: Generally do not react with dilute acid. Example: N/A
Reaction with Base:
Metal: Metal + Base → Hydrogen gas + Salt Example: Aluminium + Sodium hydroxide → Sodium aluminate + Hydrogen
Displacement Reaction: When a more reactive metal reacts with the salt solution of a less reactive metal, the more reactive metal displaces the less reactive metal from its solution, as shown in the following equation:
Metal A + Salt Solution of Metal B → Salt Solution of Metal A + Metal B
In this equation, Metal A is more reactive than Metal B.
For example, when Aluminium metal is dipped in the solution of Copper sulphate, Aluminium displaces Copper from the solution, forming Aluminium sulphate and Copper:
2Al + 3CuSO4 → Al2(SO4)3 + 3Cu
In this reaction, Aluminium is more reactive than Copper, which is why it replaces Copper from the solution of Copper sulphate.
However, when Copper metal is dipped in the solution of Aluminium nitrate, no reaction takes place, as Copper is less reactive than Aluminium.
Conclusion
In conclusion, we can say that the study of Metals and Non-Metals is an important topic for Class 10 students. The Metals and Non-Metals Class 10 Notes provide a comprehensive understanding of the physical and chemical properties of these elements. By studying these notes, students can learn about the atomic structures and reactions of metals and non-metals. They can also explore the various applications of these elements in different industries and their everyday lives. Overall, the Metals and Non-Metals Class 10 Notes serve as an essential foundation for further studies in Chemistry and Material Science. Therefore, it is crucial for students to pay attention to these notes and grasp the fundamental concepts of metals and non-metals.
Metals and Non-Metals Class 10 Notes
Download the eSaral App for complete Class 10 Video lectures, Study material, revision and much more.
