
NCERT Solutions for Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers PDF
Hey, are you a class 12 student and looking for ways to download NCERT Solutions for Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers PDF? If yes. Then read this post till the end.In this article, we have listed NCERT Solutions for Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers in PDF that are prepared by Kota’s top IITian Faculties by keeping Simplicity in mind.
If you want to learn and understand class 12 Chemistry Chapter 11 "Alcohols, Phenols and Ethers" in an easy way then you can use these solutions PDF.
NCERT Solutions helps students to Practice important concepts of subjects easily. Class 12 Chemistry solutions provide detailed explanations of all the NCERT questions that students can use to clear their doubts instantly.
If you want to score high in your class 12 Chemistry Exam then it is very important for you to have a good knowledge of all the important topics, so to learn and practice those topics you can use eSaral NCERT Solutions.
In this article, we have listed NCERT Solutions for Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers PDF that you can download to start your preparations anytime.
So, without wasting more time Let’s start.
Download NCERT Solutions for Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers PDF
Question 1. Classify the following as primary, secondary and tertiary alcohols:
(i)
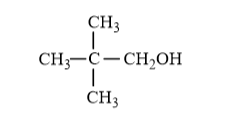
(ii) $\mathrm{H}_{2} \mathrm{C}=\mathrm{CH}-\mathrm{CH}_{2} \mathrm{OH}$
(iii) $\mathrm{CH}_{3}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{OH}$
(iv)

(v)
(vi)
Solution: (i)

OH is attached with primary carbon. So, it is primary alcohol.
(ii)
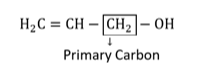
$-$ OH attached with primary carbon. So, it is primary alcohol
(iii)
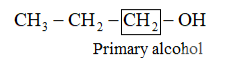
−OH is attached with primary carbon so it is primary alcohol . (iv)

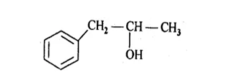
−OH group is attached with secondary carbon. So, it is secondary alcohol.
(v)
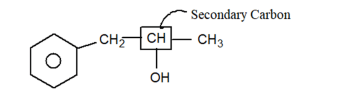
-OH group is attached with secondary carbon. So, it is secondary -alcohol
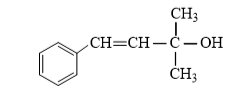
(vi)
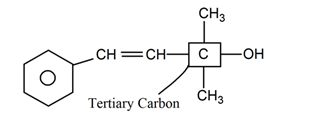
−OH group is attached with tertiary carbon. So, it is tertiary -alcohol
Primary alcohols: (i), (ii), (iii)
Secondary alcohols: (iv) and (v)Tertiary alcohols: (vi)
Question 2. Identify allylic alcohols in the above examples.
Solution. In allylic alcohols, the $-0 \mathrm{H}$ group is attached to carbon next to the carbon-carbon double bond.
Therefore, alcohols given in
(ii)
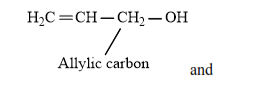
(vi)

Question 3. Name the following compounds according to IUPAC system.
(i)
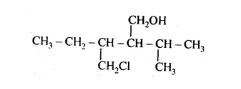
(ii)
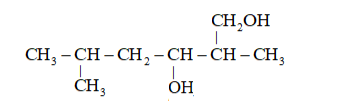
(iii)
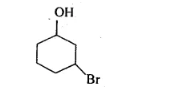
(iv)
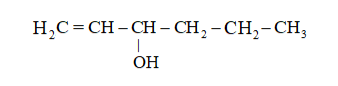
(v)
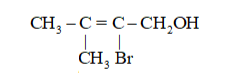
Solution: (i)
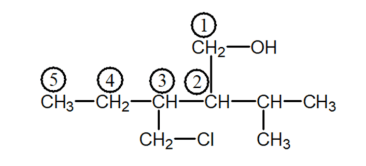
Parent Carbon $-$ chain $=5$ members(pent)
Many functional group $=-0 \mathrm{H}$ and suffix $=\mathrm{ol}$
Substituent $-\mathrm{CH}_{2} \mathrm{Cl}=$ Chloromethyl and

IUPAC name: 3-Chloromethyl-2-isopropylpentan-1-0l
(ii)

Parent Carbon Chain $=6$ members (hex)
Multi function group $=-0 \mathrm{H}$ and suffix $=\mathrm{ol}$
Substituents $=-\mathrm{CH}_{3}$ (Methyl) IUPAC name: 2,5 -Dimethylhexane-1, 3 -diol
(iii)

Parent Carbon chain ring $\rightarrow 6$ members (cyclohex)
Main functional group $=-\mathrm{OH}$ and suffix $-\mathrm{ol}$
Substituent $=-\mathrm{Br}$ (Prefix $=$ bromo $)$
IUPAC name: 3 -Bromocyclohexanol
(iv)

Main Carbon chain $=6-$ carbon (hex)
Primary Suffix $=$ ene,
main function group $=-0 \mathrm{H}$ (secondary Suffix $=\mathrm{ol}$ )
IUPAC name: Hex $-1-$ en $-3-$ ol
(v)

Main carbon $-$ chain $=4($ but $)$
Main functional group $=-0 \mathrm{H}$ (secondary Suffix $=\mathrm{ol}$ )
Substituent $-\mathrm{Br} \Rightarrow$ prefix $=$ bromo
$-\mathrm{CH}_{3}=$ methyl
Numbering should start from that carbon such that $-0 \mathrm{H}$ group gets the least number.
IUPAC name: $2-$ Bromo $-3-$ methylbut $-2-$ en $-1-$ ol
Question 4. Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanol?
(i)

(ii)

Solution.
 (i)
(i)



(ii)


$\mathrm{R}-\mathrm{MgX}$ where

Question 5. Write structures of the products of the following reactions:
(i) $\mathrm{CH}_{3}-\mathrm{CH}=\mathrm{CH}_{2} \stackrel{\mathrm{H}_{2} \mathrm{O} / \mathrm{H}^{+}}{\longrightarrow}$
(ii)

(iii)

Solution.

(i)

(ii) $\mathrm{NaBH}_{4}$ reduces pure carbonyl (ketone) into $2^{\circ}$ Alcohol

(iii) $\mathrm{NaBH}_{4}$ reduces pure carbonyl (aldehyde) into $1^{\circ}$ alcohol.
Question 6. Give structures of the products you would expect when each of the following alcohol reacts with
(a) $\mathrm{HCl}-\mathrm{ZnCl}_{2}$
(b) $\mathrm{HBr}$ and
(c) $\mathrm{SOCl}_{2}$
(i) $\quad$ Butan $-1-o l$
(ii) $2-$ Methylbutan $-2-$ ol
Solution: (a) Rate of reaction with Lucas' reagent is $3^{\circ}$ Alcohol $>2^{\circ}$ Alcohol $>$ $1^{\circ}$ alcohol.

(i) Primary alcohols do not react appreciably with Luca's reagent $\left(\mathrm{HCl}-\mathrm{ZnCl}_{2}\right)$ at room temperature. Because reaction with Luca's reagent is followed by $\mathrm{S}_{\mathrm{N}} 1$ mechanism.

(ii) Tertiary secondary alcohols react with Luca's reagent because reaction with Luca's reagent is followed by $\mathrm{S}_{\mathrm{N}} 1$ mechanism gives text in 5 to 10 minutes.

(iii) Tertiary alcohols react immediately with Luca's reagent because reaction with Luca's reagent is followed by $\mathrm{S}_{\mathrm{N}} 1$ mechanism

carbocation is $\left(3^{\circ}\right)$ which is stable due to 8 hyperconjugations and +I of the alkyl group. So, it gives turbidity immediately.

(b) (i)

(ii)

(c) (i)

(ii) Question 7. Predict the major product of acid catalysed dehydration of
(i) 1-methylcyclohexanol and (ii) butan-1-ol
Solution.

(i) In the above reaction, $\beta-\mathrm{H}$ will get eliminated and major product formed should be more stable alkene.

(ii)
Question 8. Ortho and para nitrophenols are more acidic than phenol. Draw the resonance structures of the corresponding phenoxide ions.
Solution: Resonance structure of the phenoxide ion

Resonance structures of p-nitrophenoxide ion


Resonance structures of o-nitrophenoxide ion

After losing $\mathrm{H}^{+}$ion, the phenoxide ion is formed. In ortho and meta nitro group substituted, phenoxide ions are stabilised due to $(-\mathrm{M} / \mathrm{electro}$ withdrawing) effect of $-\mathrm{NO}_{2}$ group. Hence, the conjugate base (anion) is stable and acidic character increases.
Question 9. Write the equations involved in the following reactions:
(i) Reimer - Tiemann reaction
(ii) Kolbe’s reaction
Solution: (i) Reimer - Tiemann reaction: It is the $\epsilon^{\oplus}$ substitution reaction
Reactant $=$ Phenol
Reagent $=\mathrm{CHCl}_{3}$ or aq. $\mathrm{NaOH}$

(ii) Kolbe’s reaction

Question 10. Write the reactions of Williamson synthesis of $2-$ ethoxy $-3-$ methyl pentane starting from ethanol and $3-$ methyl pentane $-2-$ ol.
Solution: An alkyl halide reacts with an alkoxide ion in Williamson's synthesis. Also, it is an $\mathrm{S}_{\mathrm{N}} 2$ reaction. In this reaction, alkyl halides should be primary having the least steric hindrance. Hence, an alkyl halide is obtained from ethanol and alkoxide ion from $3-$ methylpentan $-2-$ ol.
$\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH} \stackrel{\mathrm{HBr}}{\longrightarrow} \quad \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{Br}$
Ethanol substitution Bromoethane

Question 11. Which of the following is an appropriate set of reactants for the preparation of 1 methoxy-4-nitrobenzene and why?
(i)

(ii)

Solution: Set (ii) is an appropriate set of reactants for the preparation of 1 -methoxy-4nitrobenzene.

In set (i), sodium methoxide $\left(\mathrm{CH}_{3} \mathrm{ONa}\right)$ is a strong nucleophile as well as a strong base. Hence, an elimination reaction predominates over a substitution reaction.
Question 12. Predict the products of the following reactions:
(i) $\mathrm{CH}_{3}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{O}-\mathrm{CH}_{3}+\mathrm{HBr} \rightarrow$
(ii)

(iii)

(iv)
$\left(\mathrm{CH}_{3}\right)_{3} \mathrm{C}-\mathrm{OC}_{2} \mathrm{H}_{5} \stackrel{\mathrm{HI}}{\longrightarrow}$
Solution: (i)

(ii) Reaction proceeded by $\mathrm{S}_{\mathrm{N}} 2$ mechanism. So, $\mathrm{Br}^{-}$attacks on the more hindered group.

(iii) $\rightarrow \mathrm{OC}_{2} \mathrm{H}_{5}$ is $+\mathrm{M}$ group. So, it is a ring activating group for the attack of electrophile and $0 / p$ directing and product $p>0$ because hinderance at ortho is more than para.
(iv)

Exercise:
Question 1. Write IUPAC names of the following compounds:
(i)

(ii)

(iii)

(iv)

(v)

(vi)

(vii)

(viii)

(ix)

(x)

(xi)

Solution. (i)

Parent carbon chain $=5$ (pent)
Primary suffix $=$ ane
Substituent $=-\mathrm{CH}_{3}$ (methyl)
Main functional group $=-0 H:$ secondary suffix $=o l$
$-$ OH group should get least number
IUPAC name: $2,2,4$, trimethylpentan-3-ol
(ii)

Parent carbon Chain $=7$ (hept)
Primary suffix $=$ ane
Main functional group $=-0 \mathrm{H} ;$ Secondary suffix $=$ ol
$-0 H$ group should get least number
IUPAC name: $5-$ Ethylheptane $-2,4-$ diol
(iii)

Parent carbon chain $=4$
Primary suffix $=$ ane
Main functional group $=-\mathrm{OH}$
Secondary suffix $=$ ol
$-$ OH group should get least number
IUPAC name: butane-2, $3-$ diol
(iv)

Parent carbon chain $=3$
Primary suffix $=$ ane
Main functional group $=-0 H$; Secondary suffix $=$ ol
$-$ OH group should get least number
IUPAC name: Propane-1,2,3-triol
(v)

Main functional group $=$ Phenol
Substituent $=-\mathrm{CH}_{3}$ (methyl)
$-$ OH group should get least number
IUPAC name: 2 -Methylphenol
(vi)

Main functional group $=$ Phenol
Substituent $=-\mathrm{CH}_{3}$ (methyl)
- OH group should get least number
IUPAC name: 4-Methylphenol
(vii)

Main functional group $=$ Phenol
Substituent $=-\mathrm{CH}_{3}$ (methyl)
- OH group should get least number
IUPAC name: 2,5 -Dimethylphenol
(viii)

Main functional group $=$ Phenol
Substituent $=-\mathrm{CH}_{3}$ (methyl)
$-0 H$ group should get least number
IUPAC name: 2,6 -Dimethylphenol
(ix)

(x)

(xi) Ethoxy benzene

$2-$ Ethoxybutane
Question 2. Write structures of the compounds whose IUPAC names are as follows: (i) $\quad$ 2-Methylbutan-2-ol
(ii) 1-Phenylpropan-2-ol
(iii) 3,5 -Dimethylhexane $-1,3,5$-triol’
(iv) $2,3-$ Diethylphenol
(v) 1 – Ethoxypropane
(vi) 2-Ethoxy-3-methylpentane
(vii) Cyclohexylmethanol
(viii) 3-Cyclohexylpentan-3-ol
(ix) Cyclopent-3-en-1-ol
(x) 4-Chloro-3-ethylbutan-1-ol.
Solution. (i) $\quad$ 2-Methylbutan-2-ol

(ii) 1-Phenylpropan-2-ol

(iii) 3,5 -Dimethylhexane $-1,3,5$-triol

(iv) $2,3-$ Diethylphenol

(v) $1-$ Ethoxypropane

Functional group $=$ Ether
(vi) 2-Ethoxy-3-methylpentane

(vii) Cyclohexylmethanol

(viii) 3-Cyclohexylpentan-3-ol

(ix) Cyclopent-3-en-1-ol

(x) $\quad$ 4-Chloro-3-ethylbutan-1-ol.

The above name of the structure is wrong according to IUPAC. Correct name is 3 -methylchloro-pent-1-ol.
Question 3. (i) Draw the structures of all isomeric alcohols of molecular formula $\mathrm{C}_{5} \mathrm{H}_{12} \mathrm{O}$ and give their IUPAC names.
(ii) Classify the isomers of alcohols of molecular formula $\mathrm{C}_{5} \mathrm{H}_{12} \mathrm{O}$ (i) as primary, secondary and tertiary alcohols.
Solution: (i) The structures of all isomeric alcohols of molecular formula, $\mathrm{C}_{5} \mathrm{H}_{12} \mathrm{O}$ are shown below:
Structural isomers:- Compounds that have different structural formula but the same molecular formula and different IUPAC name are called structural isomers.
(a)

(b) Pentan-1-ol $\left(1^{\circ}\right.$ alcohol $)$

(c) 2-Methylbutan-1-ol $\left(1^{\circ}\right.$ alcohol $)$

(d) 3-Methylbutan-1-ol $\left(1^{\circ}\right.$ alcohol $)$

(e) 2,2 -Dimethylpropan-1-ol (1o alcohol)

(f) Pentan-2-ol $\left(2^{0}\right.$ alcohol $)$

(g) 3-Methylbutan-2-ol $\left(2^{\circ}\right.$ alcohol $)$

(h) Pentan-3-ol $\left(2^{0}\right.$ alcohol $)$

2-Methylbutan-2-ol $\left(3^{0}\right.$ alcohol $)$
(ii) Primary alcohol: $-\mathrm{OH}$ group attached with $1^{\mathrm{O}}$-carbon
Pentan-1-ol; $\quad$ 2-Methylbutan-1-ol; $\quad$ 3-Methylbutan-1-ol; $\quad 2,2$ Dimethylpropan-1-ol
Secondary alcohol: $-0$ H group attached with $2^{\circ}$-carbon
Pentan-2-ol; 3-Methylbutan-2-ol; Pentan-3-ol
Tertiary alcohol: $-0 H$ group attached with $3^{\circ}$-carbon
2-methylbutan-2-ol
Question 4. Explain why propanol has a higher boiling point than that of the hydrocarbon, butane?
Solution. Propanol undergoes intermolecular H-bonding because of the presence of a $-\mathrm{OH}$ group. On the other hand, butane does not form H-bonding. Butane is bonded by Van der waal's forces which is weaker than H-bond.

Therefore, extra energy is required to break hydrogen bonds. For this reason, propanol's boiling point is higher than the hydrocarbon butane.
Question 5. Alcohols are comparatively more soluble in water than hydrocarbons of comparable molecular masses. Explain this fact.
Solution: Alcohols form hydrogen bonds with water due to the presence of $-0 H$ group since $\mathrm{H}$ has $+\delta$ and 0 has lone pair. However, hydrocarbons cannot form $\mathrm{H}$-bonds with water.

Therefore, alcohols are more soluble in water than hydrocarbons of comparable molecular masses.
Question 6. What is meant by hydroboration-oxidation reaction? Illustrate it with an example.
Solution: The addition of borane and followed by oxidation is known as the hydroborationoxidation reaction.
Example: Propan-1-ol is formed by the hydroboration-oxidation reaction of the compound propene. In this reaction, propene reacts with diborane $\left(\mathrm{BH}_{3}\right)_{2}$ to form trialkyl borane as an electrophilic addition product. This electrophilic addition is
followed by anti-Markownikoff rule. This electrophilic addition product is oxidized to alcohol by hydrogen peroxide in the presence of aqueous sodium hydroxide.

Question 7. Give the structures and IUPAC names of monohydric phenols of molecular formula, $\mathrm{C}_{7} \mathrm{H}_{8} \mathrm{O}$
Solution: The isomers of monohydric phenols having the molecular formula $\mathrm{C}_{7} \mathrm{H}_{8} \mathrm{O}$ are given below:

Question 8. While separating a mixture of ortho and para nitrophenols by steam distillation, name the isomer which will be steam volatile. Give reason.
Solution. Intramolecular H-bonding is present in o-nitrophenol while in p-nitrophenol, the molecules are strongly associated due to the presence of intermolecular bonding. Hence, due to intramolecular H-bond, o-nitrophenol is steam volatile.

Question 9. Give the equations of reactions for the preparation of phenol from cumene.
Solution. To prepare phenol from cumene, first, the cumene is oxidised to cumene hydroperoxide in the presence of air $\left(\mathrm{O}_{2}\right)$ by a free radical mechanism at a temperature of $368 \mathrm{~K}-408 \mathrm{~K}$.

Then, this cumene hydroperoxide is treated with dilute acid to prepare phenol and acetone.

Question 10. Write the chemical reaction for the preparation of phenol from chlorobenzene.
Solution. Chlorobenzene is fused with $\mathrm{NaOH}$ under the conditions of $623 \mathrm{~K}$ temperature and 320 atm pressure to produce sodium phenoxide, which then gives phenol on acidification.

Question 11. Write the mechanism of hydration of ethene to yield ethanol.
Solution. The mechanism of hydration of ethene to yield ethanol involves three steps:
Step 1:
Protonation of ethene to form carbocation by electrophilic attack of $\mathrm{H}_{3} \mathrm{O}^{+}:$
$\mathrm{H}_{2} \mathrm{O}+\mathrm{H}^{+} \stackrel{\text { acid-base reaction }}{\longrightarrow} \mathrm{H}_{3} \mathrm{O}^{+}$(electroplile)


Step 2:
Nucleophilic attack of water on carbocation: lone pair of oxygen (from water, $\mathrm{H}_{2} \mathrm{O}$ ) attacks the carbocation.

Step 3:
Deprotonation to form ethanol:

Question 12. You are given benzene, conc. $\mathrm{H}_{2} \mathrm{SO}_{4}$ and $\mathrm{NaOH}$. Write the equations for the preparation of phenol using these reagents.
Solution: (i) The reaction of Benzene with concentrated sulphuric acid is called sulphonation. It is followed by electrophilic substitution.

Question 13. Show how will you synthesize:
(i) 1-Phenylethanol from a suitable alkene.
(ii) Cyclohexylmethanol using an alkyl halide by an $\mathrm{S}_{\mathrm{N}} 2$ reaction.
(iii) Pentan-1-ol using a suitable alkyl halide?
Solution: (i) By acid-catalyzed hydration of ethylbenzene (styrene), 1-phenylethanol can be synthesized by the electrophilic addition reaction.

(ii) When chloromethylcyclohexane is treated with sodium hydroxide, $\mathrm{S}_{\mathrm{N}} 2$ reaction takes place and cyclohexylmethanol is obtained.

(iii) When 1 -chlopropentane is treated with $\mathrm{NaOH}$, pentan-1-ol is produced by $\mathrm{S}_{\mathrm{N}} 2$ reaction mechanism.

Question 14. Give two reactions that show the acidic nature of phenol. Compare acidity of phenol with that of ethanol.
Solution. The acidic nature of phenol can be represented by the following two reaction:
(i) Phenol reacts with sodium to give sodium phenoxide, liberating $\mathrm{H}_{2}$ gas.

(ii) Phenol reacts with sodium hydroxide base to give sodium phenoxide salt and water as side products. This reaction exhibits the acidic nature of phenol.

Acidic character depends on the stability of anion. Anion is formed after losing $\mathrm{H}^{\oplus}$. The acidity of phenol is more than that of ethanol. This is because after losing a proton, the phenoxide ion undergoes resonance and gets stabilized.

Ethanol forms $\mathrm{CH}_{3}-\mathrm{CH}_{2} \mathrm{O}^{-}$anion which is less stable due to $+\mathrm{I}$ effect of $\mathrm{CH}_{3}-\mathrm{CH}_{2}-$. Thus, phenoxide ion is more stable than ethoxide ion.
Question 15. Explain why is ortho nitrophenol more acidic than ortho methoxyphenol?
Solution. The nitro-group is an electron-withdrawing group (-M group). The presence of nitro group in the ortho position decreases the electron density in the $0-\mathrm{H}$ bond. Thus, it is easier to lose a proton. Also, the o-nitrophenoxide ion formed after the loss of protons is stabilized by resonance. Hence, ortho nitrophenol is a stronger acid. Because the anion formed is more stable. So acidic (+M) character increases.

On the other hand, methoxy group is an electron-releasing group. As a result, it increases the electron density in the $0-\mathrm{H}$ bond, and hence, the proton cannot be given easily. Because the conjugate base is stable. Hence acidic character decreases.

Due to, this reason, ortho-nitrophenol is more acidic than ortho-methoxyphenol.
Question 16. Explain how does the $-$ OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
Solution. The $-$ OH group is an electron-donating group (+M group). Thus, it increases the electron density in the benzene ring at ortho and para as shown in the given resonance structure of phenol.

$-0 H$ group activates the ring for the electrophile to come at ortho and para.
As a result, the benzene ring is activated towards electrophilic substitution.
Question 17. Give equations of the following reactions:
(i) Oxidation of propan-1-ol with alkaline $\mathrm{KMnO}_{4}$ solution.
(ii) Bromine in $\mathrm{CS}_{2}$ with phenol.
(iii) Dilute $\mathrm{HNO}_{3}$ with phenol.
(iv) Treating phenol with chloroform in presence of aqueous $\mathrm{NaOH}$.
Solution: (i) Oxidation of propan-1-ol with alkaline $\mathrm{KMnO}_{4}$ solution gives propanoic acid.

(ii) Bromination of phenol: Reaction of phenol with $\mathrm{Br}_{2}$ in $\mathrm{CS}_{2}$. $\mathrm{Br}^{+}$is electrophilic and phenol show electrophilic substitution react with
electrophilic $\left(\stackrel{\oplus}{\mathrm{N} O}_{2}\right) \cdot-\mathrm{OH}$ group is ring activating group. It increases the
electron density at ortho and para position. Hence, the electrophilic attack occurs at ortho and para. Para is the major product than ortho because there is less hindrance at para.

(iii) Nitration of phenol


(iv)
Question 18. Explain the following with an example.
(i) Kolbe's reaction.
(ii) Reimer-Tiemann reaction.
(iii) Williamson ether synthesis.
(iv) Unsymmetrical ether.
Solution. (i) Kolbe’s reaction.
When phenol is treated with sodium hydroxide, sodium phenoxide is produced. This sodium phenoxide when treated with carbon dioxide, followed by acidification, undergoes electrophilic substitution to give ortho-hydroxybenzoic acid as the main product. This reaction is known as Kolbe's reaction.

(ii) Reimer-Tiemann reaction.
When phenol is treated with chloroform $\left(\mathrm{CHCl}_{3}\right)$ in the presence of sodium hydroxide, a -CHO group is introduced at the ortho position of the benzene ring.

When phenol is treated with chloroform $\mathrm{CHCl}_{3}$ is presence of potassium hydroxide, a -CHO group is introduced at the otho position of the benzene ring.

(iii) Williamson ether synthesis.
Williamson ether synthesis is a method to prepare symmetrical and unsymmetrical ethers by allowing alkyl halides to react with sodium alkoxides.

This reaction involves $S_{N} 2$ attack of the alkoxide ion on the alkyl halide. Better results are obtained when primary alkyl halides are used.


If the alkyl halide is secondary or tertiary, then elimination competes over substitution.
(iv) Unsymmetrical ether:
An unsymmetrical ether is an ether where both alkyl groups on the two sides of an oxygen atom differ (i.e., have an unequal number of carbon alkyl atoms). For example: ethyl methyl ether $\left(\mathrm{CH}_{3}-\mathrm{O}-\mathrm{CH}_{2} \mathrm{CH}_{3}\right)$.
Question 19. Write the mechanism of acid dehydration of ethanol to yield ethene.
Solution. The mechanism of acid dehydration of ethanol to yield ethene involves the following three steps:
Step 1:
Protonation of ethanol to form ethyl oxonium ion:

Step 2:
Formation of carbocation (rate determining step):

Carbocation is the intermediate formed in the reaction, hence carbocation rearrangement is possible during the reaction.
Step 3:
Elimination of a proton to form ethene:

The acid consumed in step 1 is released in step 3. After the formation of ethene, it is removed to shift the equilibrium in a forward direction.
Question 20. How are the following conversions carried out?
(i) Propene $\rightarrow$ Propan-2-ol.
(ii) Benzyl chloride $\rightarrow$ Benzyl alcohol.
(iii) Ethyl magnesium chloride $\rightarrow$ Propan-1-ol.
(iv) Methyl magnesium bromide $\rightarrow 2$-Methylpropan-2-ol.
Solution. (i) If propene is allowed to react with water in the presence of an acid as a catalyst, then propan-2-ol is obtained by electrophilic addition reaction:

(ii) If benzyl chloride is treated with $\mathrm{NaOH}$ (followed by acidification) then benzyl alcohol is produced.

(iii) When ethyl magnesium chloride is treated with methanal, an adduct is the produced which gives propan-1-ol on hydrolysis.

(iv) When methyl magnesium bromide is treated with propanon, an adduct is the product which gives 2-methylpropane-2-ol on hydrolysis.

Question 21. Name the reagents used in the following reactions:
(i) Oxidation of a primary alcohol to carboxylic acid.
(ii) Oxidation of a primary alcohol to aldehydes.
(iii) Bromination of phenol to 2,4,6-tribromophenol.
(iv) Benzyl alcohol to benzoic acid.
(v) Dehydration of propan-2-ol to propene.
(vi) Butan-2-one to butan-2-ol.
Solution. (i) Acidified potassium permanganate
Acidified potassium dichromate $\left(\mathrm{K}_{2} \mathrm{Cr}_{3} \mathrm{O}_{7}\right)$ and acidified potassium permanganate $\left(\mathrm{KMnO}_{4}\right)$

(ii) Pyridinium chlorochromate (PCC)
Mild oxidizing agent like pyridium chloro chromate (PCC), pyridium dichloro chromate (PDC) or $\mathrm{CrO}_{3}$ anhydrous convert primary alcohol into aldehyde.

(iii) Bromine water:
Bromination of phenol is taking place in presence of $\mathrm{Br}_{2} / \mathrm{H}_{2} \mathrm{O}$ (bromine water) and so $2,4,6$-tribromophenol formed.
(iv) Acidified potassium permanganate

Acidifies potassium permanganate convert benzylic $0, H$, bond into $-C O O H$ group.
(v) $85 \%$ phosphoric acid
Dehydration of propan-2-ol to propane is followed by elimination (E1) and reagent used is concentration $\mathrm{H}_{2} \mathrm{SO}_{4}, 85 \%$ phosphoric acid.
(vi) $\mathrm{NaBH}_{4}$ or $\mathrm{LiAIH}_{4}$
Butan-2-one to butan-2-ol

By sodium borohydride $\left(\mathrm{NaBH}_{4}\right)$, Lithium aluminium hydride $\left(\mathrm{LiAlH}_{4}\right)$ or by Na/EtOH (Bouveault-Blanc reduction)
Question 22. Give reason for the higher boiling point of ethanol in comparison to methoxymethane.
Solution. Boiling point depends on force of attraction.
Ethanol undergoes intermolecular H-bonding due to the presence of $-\mathrm{OH}$ group, resulting in the association of molecules. Extra energy is required to break these hydrogen bonds. On the other hand, methoxymethane does not undergo H-bonding. It is bonded by vanderwall force of attraction (dipole -dipole attraction). Vander wall's force is weaker than H-bond. Hence, the boiling point of ethanol is higher than that of methoxymethane.

Hydrogen bonding in ethanol. Question 23. Give IUPAC names of the following ethers:
(i)

(ii) $\mathrm{CH}_{3} \mathrm{OCH}_{2} \mathrm{CH}_{2} \mathrm{Cl}$
(iii) $\mathrm{O}_{2} \mathrm{~N}-\mathrm{C}_{6} \mathrm{H}_{4}-\mathrm{OCH}_{3}(\mathrm{p})$
(iv) $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{OCH}_{3}$
(v)

(vi)

Solution: (i)

1-Ethoxy-2-methylpropane
(ii)

2-Chloro-1-methoxyethane
(iii)

4-Nitroanisole
(iv)

1-Methoxynronane
(v)

1-Ethoxy-4,4-dimethylcyclohexane
(vi)

Ethoxybenzene
Question 24. Write the names of reagents and equations for the preparation of the following ethers by Williamson's synthesis:
(i) 1-Propoxypropane
(ii) Ethoxybenzene
(iii) 2-Methoxy-2-methylpropane
(iv) 1 -Methoxyethane
Solution: (i)

(ii)

(iii)

(iv)

Question 25. Illustrate with examples the limitations of Williamson synthesis for the preparation of certain types of ethers.
Solution. The reaction of Williamson synthesis involves $S_{N} 2$ attack of an alkoxide ion on a primary alkyl halide.

If secondary or tertiary alkyl halides are taken in place of primary alkyl halides, then elimination would compete over substitution. This is because alkoxides are nucleophiles as well as strong bases. Hence, they react with alkyl halides having $(\beta-\mathrm{H})$ which results in an elimination reaction. Alkenes are formed as a product.

Question 26. How is 1 -propoxypropane synthesized from propan-1-ol? Write mechanism of this reaction.
Solution. 1-propoxypropane can be synthesized from propan-1-ol by dehydration.
Propan-1-ol undergoes dehydration in the presence of protic acids (such as $\mathrm{H}_{2} \mathrm{SO}_{4}, \mathrm{H}_{3} \mathrm{PO}_{4}$ ) to give 1-propoxypropane.

The mechanism of this reaction involves the following three steps:
Step 1: Protonation
$\mathrm{H}^{+}$(acidic) attack on lone pair on oxygen by lewis acid-base reaction.

Step 2: Nucleophilic attack

Step 3: Deprotonation

Question 27. Preparation of ethers by acid dehydration of secondary or tertiary alcohols is not a suitable method. Give reason.
Solution. The formation of ethers by dehydration of alcohol is a bimolecular reaction $\left(\mathrm{S}_{\mathrm{N}} 2\right)$ involving the attack of an alcohol molecule on a protonated alcohol molecule. In the method, the alkyl group should be unhindered. In case of secondary or tertiary alcohols, the alkyl group is hindered. As a result, elimination dominates substitution. Hence, in place of ethers, alkenes are formed.
Question. 28. Write the equation of the reaction of hydrogen iodide with:
(i) 1 -propoxypropane
(ii) methoxybenzene and
(iii) benzyl ethyl ether.
Solution. (i)

(ii)

(iii)

Question 29. Explain the fact that in aryl alkyl ethers
(i) the alkoxy group activates the benzene ring towards electrophilic substitution and
(ii) it directs the incoming substituents to ortho and para positions in benzene ring.
Solution.

Aryl alkyl ether
In aryl alkyl ethers, due to the $+\mathrm{R}$ effect of the alkoxy group, the electron density in the benzene ring increases as shown in the following resonance structure.

Thus, benzene is activated towards electrophilic substitution by the alkoxy group.
(ii) It can also be observed from the resonance structures that the electron density increases more at the ortho and para positions than at the meta position. As a result, the incoming substituents are directed to the ortho and para positions in the benzene ring.

Question 30. Write the mechanism of the reaction of HI with methoxymethane.
Solution. The mechanism of the reaction of HI with methoxymethane is $\mathrm{S}_{\mathrm{N}} 2$ and it involves the following steps:
Step 1: Protonation of methoxymethane:

Step 2: Nucleophilic attack of $\mathrm{I}^{-}$:

Step 3: When HI is in excess and the reaction is carried out at a high temperature, the methanol formed in the second step reacts with another HI molecule and gets converted to methyl iodide by $\mathrm{S}_{\mathrm{N}} 2$ reaction
Chemical EquilibriumChemical Equilibrium

$\mathrm{CH}_{3}-\mathrm{O}-\mathrm{CH}_{3}+2 \mathrm{HO} \rightarrow 2 \mathrm{CH}_{3} \mathrm{I}+\mathrm{H}_{2} \mathrm{O}$
Concept insight: The reaction of ether with excess of Hydrogen halide results in the formation of alkyl halide.
Question 31. Write equations of the following reactions:
(i) Friedel-Crafts reaction – alkylation of anisole.
(ii) Nitration of anisole.
(iii) Bromination of anisole in ethanoic acid medium.
(iv) Friedel-Craft’s acetylation of anisole.
Solution. (i)

$-\mathrm{OCH}_{3}$ group is electron releasing (+M) group. So, it increases electron density at ortho and para. So, electrophilic $\mathrm{R}^{+}$(Carbonation) attack at ortho and nara. Para will he maior than ortho due fo less hindrance.
(ii)

(iii) In the presence of $\mathrm{H}_{2} \mathrm{SO}_{4}$ and $\mathrm{HNO}_{3}$, nitration will take place and electrophile $\stackrel{+}{N} O_{2}$ is formed.

(iv) Acylation

Question 32. Show how would you synthesize the following alcohols from appropriate alkenes?
(i)

(ii)

(iii)

(iv)

Solution: The given alcohols can be synthesized by applying Markovnikov's rule of acidcatalyzed hydration of appropriate alkenes.
(i)

(ii)

(iii)

Acid-catalyzed hydration of pent-2-ene also produces pentan-2-ol but along with pentan-3-ol.


Thus, the first reaction is preferred over the second one to get pentan-2-ol.
(iv)

Question 33. When 3 -methylbutan-2-ol is treated with HBr, the following reaction takes place:
Give a mechanism for this reaction.
(Hint: The secondary carbocation formed in step II rearranges to a more stable tertiary carbocation by a hydride ion shift from 3rd carbon atom.)
Solution. The mechanism of the given reaction involves the following steps:
Step 1: Protonation (acid base reaction)

Step 2: Formation of $2^{\circ}$ carbocation by the elimination of a water molecule

Step 3: Re-arrangement by the hydride-ion shift.

$3^{\circ}$ carbocation is more stable than $2^{\circ}$ carbocation due to more hyper conjugation in $3^{\circ}(8 \mathrm{HC})$
Step 4: Nucleophilic attack

Also Read,
Download PDF Class 12 Chemistry Notes Free .
Download PDF Class 12 Chemistry Book Chapterwise Free .
Download PDF Class 12 Chemistry Exemplar Chapterwise Free.
If you have any Confusion related to NCERT Solutions for Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers PDF then feel free to ask in the comments section down below.
To watch Free Learning Videos on Class 12 Chemistry by Kota’s top IITan’s Faculties Install the eSaral App
Comments
MatthewVaxia
Dec. 2, 2024, 6:35 a.m.
Retinaad Limited represents the top advertising technology company providing solutions for advertisement analytics & audience analytics within the Nigerian market.
We deliver audit, surveillance, and compliance solutions for:
- **outdoor media / digital outdoor advertising**,
- **BTL**,
- **shop-based promotions**,
- **broadcast tools**,
- **Radio**,
- **broadcast advertising**,
- **online PR**,
- **Socials**.
Our mission strives to support corporate brand custodians, advertisers, and media strategists enhance effectiveness and profitability.
Our company applies innovative technology to provide granular insights. By delivering a well-rounded framework, our team tackle key gaps of advertising efficiency, helping businesses thrive in an increasingly competitive landscape.
Michaelbooke
Dec. 1, 2024, 6:35 a.m.
일본 소비세 환급, 네오리아와 함께라면 간편하고 안전하게
일본 소비세 환급은 복잡하고 까다로운 절차로 많은 구매대행 셀러들이 어려움을 겪는 분야입니다. 네오리아는 다년간의 경험과 전문성을 바탕으로 신뢰할 수 있는 서비스를 제공하며, 일본 소비세 환급 과정을 쉽고 효율적으로 처리합니다.
1. 일본 소비세 환급의 필요성과 네오리아의 역할
네오리아는 일본 현지 법인을 설립하지 않아도 합법적인 방식으로 소비세 환급을 받을 수 있는 솔루션을 제공합니다. 이를 통해:
한국 개인사업자와 법인 사업자 모두 간편하게 환급 절차를 진행할 수 있습니다.
일본의 복잡한 서류 심사를 최소화하고, 현지 로컬 세리사와 협력하여 최적의 결과를 보장합니다.
2. 소비세 환급의 주요 특징
일본 연고가 없어도 가능: 일본에 사업자가 없더라도 네오리아는 신뢰할 수 있는 서비스를 통해 소비세 환급을 지원합니다.
서류 작성 걱정 해결: 잘못된 서류 제출로 환급이 거절될까 걱정될 필요 없습니다. 네오리아의 전문 대응팀이 모든 과정을 정밀하게 관리합니다.
현지 법인 운영자를 위한 추가 지원: 일본 내 개인사업자나 법인 운영자에게는 세무 감사와 이슈 대응까지 포함된 고급 서비스를 제공합니다.
3. 네오리아 서비스의 장점
전문성과 신뢰성: 정부로부터 인정받은 투명성과 세무 분야의 우수한 성과를 자랑합니다.
맞춤형 서포트: 다양한 사례를 통해 쌓은 경험으로 고객이 예상치 못한 어려움까지 미리 해결합니다.
로컬 업체에서 불가능한 고급 서비스: 한국인 고객을 위해 정확하고 간편한 세무회계 및 소비세 환급 서비스를 제공합니다.
4. 네오리아가 제공하는 혜택
시간 절약: 복잡한 절차와 서류 준비 과정을 전문가가 대신 처리합니다.
안심 환급: 철저한 관리와 세심한 대응으로 안전하게 환급을 받을 수 있습니다.
추가 서비스: 세무감사와 이슈 발생 시 즉각적인 지원으로 사업의 연속성을 보장합니다.
네오리아는 소비세 환급이 복잡하고 어렵다고 느껴지는 고객들에게 최적의 길잡이가 되어드립니다. 신뢰를 바탕으로 한 전문적인 서비스로, 더 이상 소비세 환급 문제로 고민하지 마세요!
Daviddib
Nov. 30, 2024, 9:58 p.m.
ST666 - Nhận Định Bóng Đá Kèo Nhà Cái Uy Tín
ST666: Địa Chỉ Lý Tưởng Cho Những Tín Đồ Cá Cược Bóng Đá
ST666 là nền tảng nhận định bóng đá và kèo nhà cái chuyên nghiệp, nơi người chơi có thể tham gia dự đoán các kèo đa dạng như cược tài xỉu, cược chấp, và cược 1x2. Với giao diện thân thiện, tỷ lệ cược minh bạch và ưu đãi hấp dẫn, ST666 đang dần trở thành lựa chọn hàng đầu của cộng đồng yêu bóng đá.
Nhận Định Kèo Nhà Cái Đa Dạng
Cược Tài Xỉu (#cuoctaixiu)
Dựa vào tổng số bàn thắng trong trận đấu, người chơi dễ dàng chọn lựa Tài (nhiều hơn tỷ lệ nhà cái đưa ra) hoặc Xỉu (ít hơn tỷ lệ).
ST666 cung cấp các phân tích chi tiết giúp người chơi đưa ra lựa chọn chính xác.
Cược Chấp (#cuocchap)
Thích hợp cho những trận đấu có sự chênh lệch về sức mạnh giữa hai đội. ST666 cung cấp tỷ lệ chấp tối ưu, phù hợp với cả người chơi mới và chuyên nghiệp.
Cược 1x2 (#cuoc1x2)
Phù hợp cho những ai muốn dự đoán kết quả chung cuộc (Thắng - Hòa - Thua). Đây là loại kèo phổ biến, dễ hiểu và có tỷ lệ cược hấp dẫn.
Ưu Đãi Hấp Dẫn Tại ST666
Nhận 160% tiền gửi lần đầu: Khi đăng ký tài khoản mới và nạp tiền, người chơi sẽ nhận được số tiền thưởng cực lớn, tăng cơ hội tham gia các kèo.
Hoàn tiền 3% mỗi ngày: Chính sách hoàn tiền giúp người chơi giảm thiểu rủi ro, thoải mái trải nghiệm mà không lo lắng nhiều về chi phí.
Vì Sao Nên Chọn ST666?
Nền Tảng Uy Tín: ST666 cam kết mang đến trải nghiệm cá cược minh bạch và an toàn.
Phân Tích Chuyên Sâu: Đội ngũ chuyên gia của ST666 luôn cập nhật nhận định mới nhất về các trận đấu, giúp người chơi đưa ra quyết định tối ưu.
Hỗ Trợ 24/7: Đội ngũ hỗ trợ chuyên nghiệp, luôn sẵn sàng giải đáp thắc mắc của người chơi.
Giao Dịch Nhanh Chóng: Nạp rút tiền linh hoạt, đảm bảo sự tiện lợi và bảo mật.
Cách Tham Gia ST666
Truy cập website chính thức của ST666.
Đăng ký tài khoản bằng thông tin cá nhân.
Nạp tiền lần đầu để nhận ưu đãi 160%.
Bắt đầu trải nghiệm cá cược với các kèo yêu thích!
Hãy đến với ST666 để tận hưởng không gian cá cược chuyên nghiệp, nhận định kèo chất lượng và những phần thưởng hấp dẫn. Tham gia ngay hôm nay để trở thành người chơi chiến thắng!
LutherBiB
Nov. 28, 2024, 11:18 a.m.
메인 서비스: 간편하고 효율적인 배송 및 구매 대행 서비스
1. 대행 서비스 주요 기능
메인 서비스는 고객이 한 번에 필요한 대행 서비스를 신청할 수 있도록 다양한 기능을 제공합니다.
배송대행 신청: 국내외 상품 배송을 대신 처리하며, 효율적인 시스템으로 신속한 배송을 보장합니다.
구매대행 신청: 원하는 상품을 대신 구매해주는 서비스로, 고객의 수고를 줄입니다.
엑셀 대량 등록: 대량 상품을 엑셀로 손쉽게 등록 가능하여 상업 고객의 편의성을 증대합니다.
재고 관리 신청: 창고 보관 및 재고 관리를 통해 물류 과정을 최적화합니다.
2. 고객 지원 시스템
메인 서비스는 사용자 친화적인 접근성을 제공합니다.
유저 가이드: 대행 서비스를 더욱 합리적으로 사용할 수 있도록 세부 안내서를 제공합니다.
운송장 조회: 일본 사가와 등 주요 운송사의 추적 시스템과 연동하여 운송 상황을 실시간으로 확인 가능합니다.
3. 비용 안내와 부가 서비스
비용 계산기: 예상되는 비용을 간편하게 계산해 예산 관리를 돕습니다.
부가 서비스: 교환 및 반품, 폐기 및 검역 지원 등 추가적인 편의 서비스를 제공합니다.
출항 스케줄 확인: 해외 배송의 경우 출항 일정을 사전에 확인 가능하여 배송 계획을 세울 수 있습니다.
4. 공지사항
기본 검수 공지
무료 검수 서비스로 고객의 부담을 줄이며, 보다 철저한 검수가 필요한 경우 유료 정밀 검수 서비스를 권장합니다.
수출허가서 발급 안내
항공과 해운 수출 건에 대한 허가서를 효율적으로 발급받는 방법을 상세히 안내하며, 고객의 요청에 따라 이메일로 전달됩니다.
노데이터 처리 안내
운송장 번호 없는 주문에 대한 새로운 처리 방안을 도입하여, 노데이터 발생 시 관리비가 부과되지만 서비스 품질을 개선합니다.
5. 고객과의 소통
카카오톡 상담: 실시간 상담을 통해 고객의 궁금증을 해결합니다.
공지사항 알림: 서비스 이용 중 필수 정보를 지속적으로 업데이트합니다.
메인 서비스는 고객 만족을 최우선으로 하며, 지속적인 개선과 세심한 관리를 통해 최상의 경험을 제공합니다.
RobertDer
Nov. 18, 2024, 6:35 a.m.
Judul: Merasakan Pengalaman Memainkan dengan "PG Slot" di Situs Kasino ImgToon.com
Dalam dunia permainan kasino online, permainan slot telah jadi salah satu permainan yang paling diminati, terutama jenis PG Slot. Di antara berbagai situs kasino online, ImgToon.com merupakan tujuan terbesar bagi peserta yang ingin menguji nasib mereka di berbagai permainan slot, termasuk beberapa kategori terfavorit seperti demo pg slot, pg slot gacor, dan RTP slot.
Demo PG Slot: Memulai Tanpa adanya Risiko
Salah satu fitur menarik yang ditawarkan oleh ImgToon.com adalah demo pg slot. Fungsi ini memberikan pemain untuk menguji berbagai jenis slot dari PG tanpa harus menempatkan taruhan uang asli. Dalam mode demo ini, Anda dapat menguji berbagai strategi dan mengetahui sistem permainan tanpa ancaman kehilangan uang. Ini adalah cara terbaik bagi pemula untuk terbiasa dengan permainan slot sebelum berpindah ke mode taruhan sebenarnya.
Mode demo ini juga memberikan Anda gambaran tentang potensi kemenangan dan bonus yang mungkin bisa Anda terima saat bermain dengan uang sebenarnya. Pemain dapat mencari permainan tanpa ragu, membuat pengalaman bermain di PG Slot semakin membahagiakan dan bebas tekanan.
PG Slot Gacor: Kesempatan Besar Mendulang Kemenangan
PG Slot Gacor adalah kata terkenal di kalangan pemain slot yang merujuk pada slot yang sedang dalam fase memberi kemenangan tinggi atau lebih sering dikenal "gacor". Di ImgToon.com, Anda dapat menemukan berbagai slot yang ada dalam kategori gacor ini. Slot ini dikenal memiliki peluang kemenangan lebih tinggi dan sering menghadiahkan bonus besar, membuatnya pilihan utama bagi para pemain yang ingin memperoleh keuntungan maksimal.
Namun, harus diingat bahwa "gacor" atau tidaknya sebuah slot dapat beralih, karena permainan slot tergantung pada generator nomor acak (RNG). Dengan bermain secara rutin di ImgToon.com, Anda bisa mengenali pola atau waktu yang tepat untuk memainkan PG Slot Gacor dan menambah peluang Anda untuk menang.
RTP Slot: Faktor Penting dalam Pemilahan Slot
Ketika mendiskusikan tentang slot, istilah RTP (Return to Player) adalah faktor yang sangat esensial untuk dihitung. RTP Slot berkaitan pada persentase dari total taruhan yang akan dikembalikan kepada pemain dalam jangka panjang. Di ImgToon.com, setiap permainan PG Slot diberi dengan informasi RTP yang jelas. Semakin tinggi persentase RTP, semakin besar peluang pemain untuk mendapatkan kembali sebagian besar dari taruhan mereka.
Dengan memilih PG Slot yang memiliki RTP tinggi, pemain dapat mengelola pengeluaran mereka dan memiliki peluang yang lebih baik untuk menang dalam jangka panjang. Ini menyebabkan RTP sebagai indikator utama bagi pemain yang mencari keuntungan dalam permainan kasino online.
Davidtaw
Nov. 15, 2024, 12:39 p.m.
Overview of Digital Currency Transaction Check and Compliance Solutions
In the current digital asset industry, ensuring transaction clarity and conformity with Anti-Money Laundering (AML) and Customer Identification standards is vital. Here is an summary of well-known platforms that provide solutions for crypto transaction monitoring, check, and resource security.
1. Tokenmetrics.com
Overview: Tokenmetrics offers digital asset assessment to evaluate potential risk threats. This solution allows investors to review coins prior to buying to avoid likely scam assets. Attributes:
- Threat assessment.
- Perfect for buyers looking to avoid hazardous or scam projects.
2. Metamask.Monitory.Center
Summary: Metamask.Monitory.Center permits individuals to review their digital asset assets for suspicious transactions and regulatory adherence. Features:
- Checks assets for “cleanliness”.
- Offers notifications about possible resource locks on certain exchanges.
- Gives detailed insights after account linking.
3. Best Change
Description: Bestchange.ru is a platform for tracking and verifying crypto trade transactions, guaranteeing transparency and transfer security. Features:
- Deal and wallet tracking.
- Restriction checks.
- Online portal; supports BTC and multiple additional digital assets.
4. AML Bot
Overview: AMLCheck Bot is a holding monitor and anti-money laundering service that utilizes machine learning models to identify questionable transactions. Features:
- Transfer monitoring and personal check.
- Accessible via internet and Telegram.
- Supports coins including BSC, BTC, DOGE, and more.
5. Alfabit AML
Overview: AlphaBit provides complete Anti-Money Laundering (AML) solutions tailored for the crypto market, assisting firms and banks in preserving standard compliance. Advantages:
- Comprehensive compliance features and screenings.
- Complies with current protection and conformity requirements.
6. AML Node
Summary: AMLNode provides compliance and identification services for digital currency companies, which includes deal observing, restriction checks, and analysis. Benefits:
- Danger analysis solutions and sanctions checks.
- Important for maintaining safe business activities.
7. Btrace AML Crypto
Summary: Btrace AML Crypto specializes in fund check, providing transfer observation, restriction evaluations, and support if you are a affected by theft. Highlights:
- Useful help for fund retrieval.
- Transaction tracking and protection options.
Exclusive USDT Validation Solutions
Our platform also evaluates different platforms offering check tools for Tether transfers and accounts:
- **USDT TRC20 and ERC20 Verification:** Many services support comprehensive screenings for USDT transactions, aiding in the finding of suspicious actions.
- **AML Validation for USDT:** Tools are provided for monitoring for money laundering activities.
- **“Cleanliness” Checks for Accounts:** Validation of deal and holding purity is available to find potential risks.
**Wrap-up**
Selecting the right service for validating and monitoring digital currency deals is essential for ensuring safety and compliance compliance. By viewing our reviews, you can choose the most suitable solution for transfer observation and resource security.
JefferyCouct
Nov. 8, 2024, 6:35 a.m.
Hercule99: Susunan 8 Aplikasi Percobaan PG Slot Tanpa Kecurangan Aman di Negeri Ini
PG Slot sudah menjadi favorit favorit utama untuk para penggemar slot di Nusantara, menawarkan ragam game yang memikat dengan kenikmatan dan kegembiraan tanpa batas. Di tengah popularitasnya, terdapat keraguan seputar kecurangan atau kecurangan dalam game online. Tetapi, Game PG sudah sukses menanggapi kekhawatiran ini dengan menghadirkan permainan slot tanpa curang yang terjamin dan terpercaya.
Berbekal teknologi keamanan modern dan fitur mutakhir, PGS memberikan jaminan bahwa semua permainan mereka adil dan aman untuk semua pemain. Inilah kumpulan 8 game demo PG Slot bebas curang yang populer dan dapat dipercaya di Nusantara:
1. Demo Fortune Mouse PG Slot
Fortuna Mouse merupakan permainan slot yang menarik perhatian dengan gambar memikat dan tema yang menggemaskan. Selain visual yang memikat, game ini dilengkapi dengan teknologi enkripsi terbaru dari PG Soft agar melindungi semua data pemain. Setiap putaran dijamin bebas dari manipulasi, sehingga pemain tidak perlu khawatir.
2. Slot Demo Dragon Hatch
Telur Naga memasukkan player ke alam khayalan yang dilengkapi keistimewaan dan kekuatan ajaib. Namun keunggulan permainan ini ada pada keamanan mutakhirnya. Menggunakan teknologi anti curang, game ini mewujudkan keadilan dalam kemenangan dengan cara fair tanpa adanya manipulasi dari luar.
3. Demo Perjalanan Menuju Kekayaan PGS
Game Journey to the Wealth memanggil player dalam petualangan menemukan kekayaan yang tak berujung. Berbekal bonus melimpah serta RTP besar, slot ini menawarkan sensasi berlipat serta kepastian keadilan pada setiap putarannya. User dapat merasakan sensasi bermain tanpa rasa cemas mengenai manipulasi.
4. Uji Coba Pulau Bikini Slot PG
Slot ini mengajak pemain dalam suasana pulau yang indah yang indah serta penuh kesenangan. Di balik kesan tenangnya, Game Bikini dibekali sistem keamanan yang andal, yang menjadikannya sebagai game Slot PG bebas manipulasi yang terpercaya. Pemain bisa bermain tanpa ada kekhawatiran curang.
5. Versi Demo Medusa II PGS
Di dalam game Medusa II, pengguna dibawa pada kisah epik bertemu monster legendaris. Gim ini menggunakan proteksi terbaru yang menjaga kejujuran tiap giliran. Dengan begitu, player dapat fokus untuk menang tanpa ada campur tangan eksternal.
6. Versi Demo Bombs PG Slot
Berbekal tema perang yang intensif, Bom Jatuh memicu semangat para pemain. Dalam ketegangan permainan, keamanan tetap prioritas utama. Pengamanan PG Soft memastikan keamanan transaksi dan hasil, menjamin keamanan untuk pemain di jalan menuju kemenangan.
7. Demo Bull Fight Pocket Games Slot
Bull Fight memasukkan player ke arena banteng yang seru. Dalam game ini, PG Soft memastikan bahwa setiap putaran memiliki keadilan dan integritas. Pengguna bisa menikmati permainan dengan adil dan aman tanpa takut akan kecurangan.
8. Percobaan Muay Thai PGS
Juara Muay Thai menawarkan tema bela diri yang menarik, membawa pemain ke dalam pertarungan sengit di atas ring. Game ini memukau dari tema, tetapi juga dalam keamanan. Tiap giliran dipantau secara ketat, menjamin pengalaman bermain yang bebas dari gangguan dan kecurangan.
Nilai Positif Main Slot Demo PG Soft dengan RTP Besar
Demo game PG Soft anti-kecurangan dikenal dengan RTP besar yang di atas standar industri. Ini memberikan peluang yang lebih baik bagi pemain untuk mendapatkan profit dari investasi. Memilih slot PG Soft tidak sekadar hiburan, tetapi juga kesempatan profit lebih tinggi.
Dengan daftar game di atas, pemain bisa menikmati slot dengan keadilan, keamanan, dan kesenangan. Teknologi modern di PG Slot menjamin tiap game berjalan lancar, memberikan rasa percaya bagi pengguna.
Charlesitern
Nov. 7, 2024, 9:43 p.m.
สล็อตไม่ผ่านเอเย่นต์คือระบบเกมผ่านเน็ตที่ให้นักเล่นเกมเข้าถึงสล็อตแมชชีนได้โดยตรงจากเว็บไซต์ โดยไม่ต้องใช้บริการจากผู้แทนหรือตัวกลางใดๆ ข้อดีของเว็บสล็อตตรงคือการป้องกันสูงขึ้น เนื่องจากผู้เล่นไม่ต้องเป็นกังวลเรื่องการเสี่ยงจากการใช้บริการผ่านเอเย่นต์ อีกทั้งยังมีการจ่ายเงินรางวัลที่มากกว่าและโบนัสมากมาย เนื่องจากไม่มีค่าธรรมเนียมจากเอเย่นต์ ทำให้นักเล่นเข้าถึงเกมสปินได้อย่างสะดวกและทันใจ พร้อมรับประสบการณ์การเล่นเกมที่ดีขึ้นและไม่ติดขัด
การหมุน สล็อตเว็บตรง ไม่เหมือนกับ สล็อตแบบดั้งเดิมอย่างไร?
สล็อตไม่ผ่านเอเย่นต์เป็นตัวเลือกที่ไม่มีการผ่านตัวแทน ทำให้ผู้เล่นสามารถเข้าถึง เกมสปินและเงินรางวัลได้โดยตรงจากผู้ดำเนินการ ลดการเสี่ยงในการเสียเปรียบหรือเสียค่าธรรมเนียมสูง นอกจากนี้ สล็อตตรงยังมีตัวเลือกที่หลากหลายของเกมสล็อตให้เลือกสรรมากกว่าในสล็อตดั้งเดิม เนื่องจากเว็บสล็อตตรงมักจะอัปเดตบ่อยและเพิ่มเกมใหม่ๆอย่างสม่ำเสมอ อัตราการจ่ายเงิน (เรทการจ่าย) ของเว็บสล็อตมักจะสูงกว่าสล็อตทั่วไป เนื่องจากไม่มีค่าธรรมเนียมพิเศษ ทำให้นักเล่นมีโอกาสรับกำไรที่สูงขึ้น อีกทั้งยัง โปรโมชั่นและโบนัสที่มากกว่า โดยเว็บสล็อตตรงมักมีข้อเสนอที่น่าสนใจและโปรแกรมสะสมแต้มที่น่าสนใจมากขึ้น
โปรโมชันและโปรโมชั่นพิเศษในเว็บสล็อตตรงที่น่าสนใจ
สล็อตเว็บตรงมักมีข้อเสนอและโปรโมชั่นพิเศษที่ดีมากสำหรับผู้เล่น เริ่มตั้งแต่โบนัสสมาชิกใหม่สำหรับผู้ที่เพิ่งสมัคร เงินเพิ่มในการฝาก เครดิตเล่นฟรี รวมถึงการสะสมคะแนนที่สามารถแลกของรางวัลหรือสิทธิประโยชน์ต่างๆได้ ทำให้นักเล่นเกมมีความคุ้มค่าและสิทธิประโยชน์มากมาย การมีโปรโมชันที่คุ้มค่าช่วยให้นักเดิมพันสามารถเพิ่มโอกาสรับรางวัลและลดเงินลงทุนในการเล่น นอกจากนี้ยังมีโปรโมชันเสริมเช่นคืนเงินบางส่วนจากยอดเสียและรางวัลพิเศษตามช่วงเทศกาลอีกด้วย
สรุปว่า สล็อตเว็บตรงเป็นทางเลือกที่คุ้มค่าสำหรับผู้ที่ต้องการ การเล่นที่ง่ายและความปลอดภัยในการเดิมพัน มีการจ่ายเงินที่มากกว่า โปรโมชั่นมากมาย และการเล่นเกมที่ลื่นไหลโดยไม่มีการผ่านเอเย่นต์
JefferyCouct
Nov. 6, 2024, 7:52 p.m.
Herkules99: Kumpulan delapan Aplikasi Versi Demo PG Slot Tanpa Kecurangan Aman di Negeri Ini
PG Slot telah menjadi kesukaan bagi banyak pemain slot di negeri ini, menyuguhkan berbagai permainan slot yang memikat dengan pengalaman dan kegembiraan tanpa henti. Dengan meningkatnya popularitasnya, terdapat keraguan akan munculnya kecurangan atau kecurangan di platform judi daring. Akan tetapi, PG Slot sudah sukses menghadapi tantangan ini dengan memperkenalkan game slot anti-rungkad yang terlindungi serta terjamin.
Berbekal teknologi keamanan modern serta fitur canggih, Game PG Slot menjamin bahwa semua permainan mereka lancar dan terlindungi untuk semua user. Berikut daftar 8 slot demo PG anti rungkad yang menguntungkan serta terpercaya di negeri ini:
1. Fortune Mouse Demo
Tikur Keberuntungan merupakan permainan slot yang memikat hati dengan gambar memikat serta tema menarik. Di luar visual yang cantik, permainan ini menggunakan enkripsi modern yang menjaga data pemain dan transaksinya. Setiap perputaran aman dari kecurangan, agar permainan tetap nyaman.
2. Slot Demo Dragon Hatch
Telur Naga memasukkan player ke semesta fiksi yang dipenuhi keistimewaan dengan energi mistis. Tetapi kelebihan utama gim ini terdapat pada fitur keamanannya. Berbekal fitur anti-manipulasi, game ini mewujudkan keadilan dalam kemenangan secara jujur bebas dari campur tangan luar.
3. Demo Journey to the Wealth PG Slot
Game Perjalanan Menuju Kekayaan membawa pengguna ke perjalanan menemukan kekayaan tanpa batas. Berbekal bonus melimpah dan RTP yang tinggi, permainan ini menghadirkan sensasi berlipat dan juga keadilan pada setiap putarannya. Pemain bisa merasakan serunya bermain tanpa rasa cemas terhadap kecurangan.
4. Uji Coba Bikini Paradise Pocket Games Slot
Permainan ini membuat pemain terjun ke suasana pulau tropis yang elok serta penuh keceriaan. Di balik nuansa santainya, Bikini Tropis memiliki fitur keamanan canggih, menjadikannya salah satu game Slot PG bebas manipulasi yang terjamin. User bisa bermain tanpa khawatir akan adanya kecurangan.
5. Uji Coba Medusa II: Misi Perseus PG Slot
Dalam Medusa II, pemain diajak dalam petualangan epik menghadapi monster legendaris. Slot ini dibekali teknologi pengaman terbaru yang memastikan setiap putaran aman. Oleh karena itu, pengguna bisa fokus mengejar kemenangan tanpa ada campur tangan eksternal.
6. Demo Bombs Pocket Games Slot
Dengan tema perang yang intens, Bombs Away memicu semangat para pemain. Di antara ketegangan bermain, fokus pada keamanan utama. Pengamanan PG Soft menjaga transaksi dan hasil game, memberikan keamanan kepada player ketika mencari kemenangan.
7. Demo Arena Banteng Pocket Games Slot
Game Bull Fight membawa pemain ke arena yang mendebarkan. Di dalam game ini, PG Soft memastikan keadilan dalam setiap giliran. Pengguna bisa menikmati permainan dengan adil dan aman tanpa rasa khawatir akan manipulasi.
8. Demo Muay Thai Champion PGS
Slot Muay Thai Champion mengusung tema bertarung yang seru, mengundang player bertarung sengit di ring. Gim ini tidak hanya seru karena tema, tapi juga dari sisi keamanan. Setiap putaran dipantau dengan ketat, menjamin pengalaman bermain yang bebas dari gangguan dan kecurangan.
Kelebihan Memilih Demo Slot PG Soft Ber-RTP Tinggi
Demo game PG Soft anti-kecurangan dikenal dengan RTP tinggi mereka yang sering kali di atas rata-rata industri. Ini memberikan peluang yang lebih baik bagi pemain untuk mendapatkan profit dari investasi. Memilih slot PG Soft tidak sekadar hiburan, namun juga potensi untung yang lebih besar.
Dengan pilihan permainan di atas, pemain bisa menikmati slot dengan keadilan, keamanan, dan kesenangan. Melalui sistem canggih dari PG Slot membuat setiap permainan aman tanpa gangguan, menambah keyakinan bagi player.
Hubertpioks
Oct. 31, 2024, 6:35 a.m.
ทดลองเล่นสล็อต PG: สัมผัสประสบการณ์เกมสล็อตออนไลน์แบบใหม่
ก่อนที่คุณจะเริ่มเล่นเกมสล็อตออนไลน์ สิ่งสำคัญคือการทำความรู้จักกับการทดลองเล่นเสียก่อน เกมสล็อต ทดลองเล่นสล็อต นั้นได้รับแรงบันดาลใจจากเครื่องสล็อตแบบดั้งเดิม โดยเฉพาะอย่างยิ่ง Triple Gold Slot ซึ่งเคยเป็นที่นิยมอย่างมากในคาสิโนต่างประเทศ ในเกมสล็อต ทดลองเล่นสล็อต PG ผู้เล่นจะได้สัมผัสกับรูปแบบของเกมที่มีความเรียบง่ายและคลาสสิก มาพร้อมกับรีล (Reel) จำนวน 5 แถวและเพย์ไลน์ (Payline) หรือรูปแบบการชนะรางวัลที่มากถึง 15 รูปแบบ ทำให้มีโอกาสชนะได้หลากหลายมากยิ่งขึ้น
สัญลักษณ์ต่าง ๆ ในเกมนี้สร้างความรู้สึกถึงบรรยากาศของสล็อตดั้งเดิม โดยมีสัญลักษณ์ที่เป็นที่รู้จักเช่น รูปเชอร์รี่ ตัวเลข 7 และเพชร ซึ่งนอกจากจะทำให้เกมมีความน่าสนใจแล้วยังเพิ่มโอกาสในการทำกำไรอีกด้วย
ความสะดวกสบายของเกมสล็อต PG
ทดลองเล่นสล็อต PG นั้นไม่เพียงแค่มีรูปแบบการเล่นที่เข้าใจง่าย แต่ยังมีความสะดวกสบายอย่างยิ่ง ไม่ว่าคุณจะใช้คอมพิวเตอร์หรือโทรศัพท์มือถือรุ่นใด เพียงแค่เชื่อมต่ออินเทอร์เน็ต คุณก็สามารถเข้าร่วมสนุกได้ทันที ทดลองเล่นสล็อต PG ยังถูกออกแบบมาให้รองรับอุปกรณ์หลากหลายประเภท เพื่อมอบประสบการณ์การเล่นที่ราบรื่นไม่ติดขัดแก่ผู้เล่นทุกคน
การเลือกธีมและรูปแบบเกม
ที่สำคัญ ทดลองเล่นสล็อต PG ยังมีหลากหลายธีมให้เลือกเล่น ไม่ว่าจะเป็นธีมที่น่าตื่นเต้น น่ารัก หรือธีมที่มีความสมจริง ทำให้ผู้เล่นสามารถสนุกสนานไปกับรูปแบบต่าง ๆ ตามความชอบ
ด้วยคุณสมบัติทั้งหมดนี้ ทดลองเล่นสล็อต PG ได้กลายเป็นตัวเลือกที่นิยมในหมู่ผู้เล่นเกมออนไลน์ที่กำลังมองหาความท้าทายใหม่ ๆ และการชนะที่ง่ายขึ้น หากคุณกำลังมองหาประสบการณ์ใหม่ ๆ การทดลองเล่นเกมสล็อตเป็นทางเลือกที่คุณไม่ควรพลาด!
Williamoweda
Oct. 30, 2024, 6:35 a.m.
RGBET – Hướng Dẫn Truy Cập Nhà Cái RGBet Chính Thức Mới Nhất 2024
Cảnh Báo Về Các Trang Web Giả Mạo RGBET.INFO
Kính gửi quý khách hàng và người dùng thân mến,
Chúng tôi, đại diện chính thức của <rgbet>co], muốn thông báo về một số trang web giả mạo, đặc biệt là rgbet.info, đang mạo danh RGBet nhằm đánh lừa người dùng. Chúng tôi khẳng định rằng rgbet.info không có bất kỳ liên kết nào với RGBet chính thức, và việc truy cập vào các trang này có thể gây nguy cơ cho thông tin cá nhân cũng như tài khoản của bạn.
Việc giả mạo thương hiệu RGBet đã ảnh hưởng không nhỏ đến uy tín của chúng tôi và tiềm ẩn nguy cơ cho khách hàng. Những trang web này thường sẽ yêu cầu người dùng cung cấp thông tin nhạy cảm như số tài khoản ngân hàng, mật khẩu, hoặc các thông tin cá nhân khác, dễ dẫn đến mất mát tài sản hoặc dữ liệu cá nhân.
Cách Nhận Biết Liên Kết Chính Thức Của RGBet
Để đảm bảo sự an toàn tuyệt đối khi tham gia RGBet, khách hàng nên xác nhận rằng mình chỉ đang truy cập trang web RGBet thông qua liên kết chính thức tại <rgbet>co]. Đây là kênh duy nhất mà RGBet cung cấp để đảm bảo tính bảo mật, an toàn cho người dùng và tránh những rủi ro không đáng có. RGBet không bao giờ chuyển hướng người dùng đến trang web bên thứ ba như da88 hoặc các trang tương tự, và chúng tôi khuyến cáo bạn không nên tin tưởng những trang này.
Hướng Dẫn Và Hỗ Trợ Chính Thức Từ RGBet
RGBet luôn cố gắng hỗ trợ tốt nhất cho khách hàng thông qua các kênh liên lạc chính thức. Mọi thắc mắc, yêu cầu hỗ trợ hoặc vấn đề gặp phải khi truy cập có thể được giải đáp bởi đội ngũ hỗ trợ của chúng tôi. Vui lòng chỉ liên hệ thông qua các phương thức chính thức tại <rgbet>co] để đảm bảo thông tin được bảo mật.
Kính mong quý khách hàng luôn cảnh giác và lựa chọn RGBet một cách an toàn và thông minh để có trải nghiệm tuyệt vời nhất.
Trân trọng,
Đại Diện RGBet
Craigstalf
Oct. 29, 2024, 6:35 a.m.
RGBET – Hướng Dẫn Truy Cập Nhà Cái RGBet Chính Thức Mới Nhất 2024
Cảnh Báo Về Các Trang Web Giả Mạo RGBET.INFO
Kính gửi quý khách hàng và người dùng thân mến,
Chúng tôi, đại diện chính thức của <rgbet>co], muốn thông báo về một số trang web giả mạo, đặc biệt là rgbet.info, đang mạo danh RGBet nhằm đánh lừa người dùng. Chúng tôi khẳng định rằng rgbet.info không có bất kỳ liên kết nào với RGBet chính thức, và việc truy cập vào các trang này có thể gây nguy cơ cho thông tin cá nhân cũng như tài khoản của bạn.
Việc giả mạo thương hiệu RGBet đã ảnh hưởng không nhỏ đến uy tín của chúng tôi và tiềm ẩn nguy cơ cho khách hàng. Những trang web này thường sẽ yêu cầu người dùng cung cấp thông tin nhạy cảm như số tài khoản ngân hàng, mật khẩu, hoặc các thông tin cá nhân khác, dễ dẫn đến mất mát tài sản hoặc dữ liệu cá nhân.
Cách Nhận Biết Liên Kết Chính Thức Của RGBet
Để đảm bảo sự an toàn tuyệt đối khi tham gia RGBet, khách hàng nên xác nhận rằng mình chỉ đang truy cập trang web RGBet thông qua liên kết chính thức tại <rgbet>co]. Đây là kênh duy nhất mà RGBet cung cấp để đảm bảo tính bảo mật, an toàn cho người dùng và tránh những rủi ro không đáng có. RGBet không bao giờ chuyển hướng người dùng đến trang web bên thứ ba như da88 hoặc các trang tương tự, và chúng tôi khuyến cáo bạn không nên tin tưởng những trang này.
Hướng Dẫn Và Hỗ Trợ Chính Thức Từ RGBet
RGBet luôn cố gắng hỗ trợ tốt nhất cho khách hàng thông qua các kênh liên lạc chính thức. Mọi thắc mắc, yêu cầu hỗ trợ hoặc vấn đề gặp phải khi truy cập có thể được giải đáp bởi đội ngũ hỗ trợ của chúng tôi. Vui lòng chỉ liên hệ thông qua các phương thức chính thức tại <rgbet>co] để đảm bảo thông tin được bảo mật.
Kính mong quý khách hàng luôn cảnh giác và lựa chọn RGBet một cách an toàn và thông minh để có trải nghiệm tuyệt vời nhất.
Trân trọng,
Đại Diện RGBet
SheldonBip
Oct. 27, 2024, 6:35 a.m.
SA Gaming เป็น บริษัท เกม บาคาร่าคลาสสิก ออนไลน์ ซึ่งได้รับความนิยม ใน ทั่วโลก ว่าเป็น หัวหน้าค่าย ในการให้บริการ บริการ คาสิโนออนไลน์ โดยเฉพาะในด้าน ไพ่ บาคาร่า ซึ่งเป็น เกมส์ ที่ นักเดิมพัน สนใจเล่นกัน ทั่วไป ใน คาสิโนจริง และ แพลตฟอร์มออนไลน์ ด้วย ลักษณะการเล่น ที่ สะดวก การเลือกเดิมพัน เพียง ฝั่ง ผู้เล่น หรือ ฝั่งเจ้ามือ และ ความเป็นไปได้ในการชนะ ที่ มีความเป็นไปได้สูง ทำให้ เกมพนันบาคาร่า ได้รับ ความสนใจ อย่างมากใน ช่วงหลายปีหลัง โดยเฉพาะใน ไทย
หนึ่งในรูปแบบการเล่น ยอดนิยมที่ เอสเอ เกมมิ่ง เสนอ คือ บาคาร่าเร็ว ซึ่ง ช่วยให้ผู้เล่น ต้องการ ความรวดเร็ว และ การเดิมพันไว สามารถ เล่นได้อย่างเร็ว นอกจากนี้ยังมีโหมด No Commission Baccarat ซึ่งเป็น โหมด ที่ ไม่ต้องเสียค่าคอมมิชชั่นเพิ่มเติม เมื่อชนะ การแทง ฝั่งเจ้ามือ ทำให้ เกมนี้ ได้รับ ความสนใจ จาก นักพนัน ที่มองหา ความคุ้มค่า ในการ ลงทุน
เกมการ์ด ของ เอสเอ เกมมิ่ง ยัง ได้รับการออกแบบ ให้มี กราฟิก และ เสียง ที่ เสมือนจริง สร้างบรรยากาศ ที่ น่าสนุก เหมือนอยู่ใน คาสิโนจริง พร้อมกับ ฟังก์ชัน ที่ทำให้ ผู้เสี่ยงโชค สามารถเลือก วิธีแทง ที่ มีให้เลือกมากมาย ไม่ว่าจะเป็น การเดิมพัน ตามเทคนิค ของตนเอง หรือการ พึ่งกลยุทธ์ เพื่อชนะ นอกจากนี้ยังมี ดีลเลอร์จริง ที่ ควบคุมเกม ในแต่ละ สถานที่ ทำให้ บรรยากาศ มี ความน่าสนใจ มากยิ่งขึ้น
ด้วย ความยืดหยุ่น ใน การเดิมพัน และ วิธีที่สะดวก ในการ เล่น SA Gaming ได้ พัฒนา เกมเสี่ยงโชค ที่ ตอบโจทย์ ทุก ชนิด ของนักเสี่ยงโชค ตั้งแต่ ผู้ที่เริ่มต้น ไปจนถึง นักเดิมพัน มืออาชีพ
Jamesinjut
Oct. 25, 2024, 7:20 a.m.
Backlinks are one technique for digital marketing enhancement. Inbound links are as to attract search robots scrapers and archivers to the site and to transfer weight and enhance authority. There are many separate approaches in this aspect. One must realize that inbound links are not consistently ideal for all subjects; based on the subject, a unique link-building plan and direction is required, by factoring in the competition in this field, topic relevance and the appropriateness of effective link integration.
Rivalry is also varied, so an supplementary approach is used as Articles and Web 2.0 platforms, as these tools are independently ranked by search engines and are helpful to build subject-aligned sites that have a low-frequency or moderate-traffic phrase in the title bar.
Normansaf
Oct. 24, 2024, 6:14 p.m.
เล่นบาคาร่าแบบรวดเร็วทันใจกับสปีดบาคาร่า
ถ้าคุณเป็นแฟนตัวยงของเกมไพ่บาคาร่า คุณอาจจะเคยชินกับการรอคอยในแต่ละรอบการเดิมพัน และรอจนดีลเลอร์แจกไพ่ในแต่ละตา แต่คุณรู้หรือไม่ว่า ตอนนี้คุณไม่ต้องรออีกต่อไปแล้ว เพราะ SA Gaming ได้พัฒนาเกมบาคาร่าโหมดใหม่ขึ้นมา เพื่อให้ประสบการณ์การเล่นของคุณน่าตื่นเต้นยิ่งขึ้น!
ที่ SA Gaming คุณสามารถเลือกเล่นไพ่บาคาร่าในโหมดที่เรียกว่า สปีดบาคาร่า (Speed Baccarat) โหมดนี้มีคุณสมบัติพิเศษและข้อดีที่น่าสนใจมากมาย:
ระยะเวลาการเดิมพันสั้นลง — คุณไม่จำเป็นต้องรอนานอีกต่อไป ในโหมดสปีดบาคาร่า คุณจะมีเวลาเพียง 12 วินาทีในการวางเดิมพัน ทำให้เกมแต่ละรอบจบได้รวดเร็ว โดยเกมในแต่ละรอบจะใช้เวลาเพียง 20 วินาทีเท่านั้น
ผลตอบแทนต่อผู้เล่นสูง (RTP) — เกมสปีดบาคาร่าให้ผลตอบแทนต่อผู้เล่นสูงถึง 4% ซึ่งเป็นมาตรฐานความเป็นธรรมที่ผู้เล่นสามารถไว้วางใจได้
การเล่นเกมที่รวดเร็วและน่าตื่นเต้น — ระยะเวลาที่สั้นลงทำให้เกมแต่ละรอบดำเนินไปอย่างรวดเร็ว ทันใจ เพิ่มความสนุกและความตื่นเต้นในการเล่น ทำให้ประสบการณ์การเล่นของคุณยิ่งสนุกมากขึ้น
กลไกและรูปแบบการเล่นยังคงเหมือนเดิม — แม้ว่าระยะเวลาจะสั้นลง แต่กลไกและกฎของการเล่น ยังคงเหมือนกับบาคาร่าสดปกติทุกประการ เพียงแค่ปรับเวลาให้เล่นได้รวดเร็วและสะดวกขึ้นเท่านั้น
นอกจากสปีดบาคาร่าแล้ว ที่ SA Gaming ยังมีโหมด No Commission Baccarat หรือบาคาร่าแบบไม่เสียค่าคอมมิชชั่น ซึ่งจะช่วยให้คุณสามารถเพลิดเพลินไปกับการเล่นได้โดยไม่ต้องกังวลเรื่องค่าคอมมิชชั่นเพิ่มเติม
เล่นบาคาร่ากับ SA Gaming คุณจะได้รับประสบการณ์การเล่นที่สนุก ทันสมัย และตรงใจมากที่สุด!
Normansaf
Oct. 24, 2024, 11:01 a.m.
เล่นบาคาร่าแบบรวดเร็วทันใจกับสปีดบาคาร่า
ถ้าคุณเป็นแฟนตัวยงของเกมไพ่บาคาร่า คุณอาจจะเคยชินกับการรอคอยในแต่ละรอบการเดิมพัน และรอจนดีลเลอร์แจกไพ่ในแต่ละตา แต่คุณรู้หรือไม่ว่า ตอนนี้คุณไม่ต้องรออีกต่อไปแล้ว เพราะ SA Gaming ได้พัฒนาเกมบาคาร่าโหมดใหม่ขึ้นมา เพื่อให้ประสบการณ์การเล่นของคุณน่าตื่นเต้นยิ่งขึ้น!
ที่ SA Gaming คุณสามารถเลือกเล่นไพ่บาคาร่าในโหมดที่เรียกว่า สปีดบาคาร่า (Speed Baccarat) โหมดนี้มีคุณสมบัติพิเศษและข้อดีที่น่าสนใจมากมาย:
ระยะเวลาการเดิมพันสั้นลง — คุณไม่จำเป็นต้องรอนานอีกต่อไป ในโหมดสปีดบาคาร่า คุณจะมีเวลาเพียง 12 วินาทีในการวางเดิมพัน ทำให้เกมแต่ละรอบจบได้รวดเร็ว โดยเกมในแต่ละรอบจะใช้เวลาเพียง 20 วินาทีเท่านั้น
ผลตอบแทนต่อผู้เล่นสูง (RTP) — เกมสปีดบาคาร่าให้ผลตอบแทนต่อผู้เล่นสูงถึง 4% ซึ่งเป็นมาตรฐานความเป็นธรรมที่ผู้เล่นสามารถไว้วางใจได้
การเล่นเกมที่รวดเร็วและน่าตื่นเต้น — ระยะเวลาที่สั้นลงทำให้เกมแต่ละรอบดำเนินไปอย่างรวดเร็ว ทันใจ เพิ่มความสนุกและความตื่นเต้นในการเล่น ทำให้ประสบการณ์การเล่นของคุณยิ่งสนุกมากขึ้น
กลไกและรูปแบบการเล่นยังคงเหมือนเดิม — แม้ว่าระยะเวลาจะสั้นลง แต่กลไกและกฎของการเล่น ยังคงเหมือนกับบาคาร่าสดปกติทุกประการ เพียงแค่ปรับเวลาให้เล่นได้รวดเร็วและสะดวกขึ้นเท่านั้น
นอกจากสปีดบาคาร่าแล้ว ที่ SA Gaming ยังมีโหมด No Commission Baccarat หรือบาคาร่าแบบไม่เสียค่าคอมมิชชั่น ซึ่งจะช่วยให้คุณสามารถเพลิดเพลินไปกับการเล่นได้โดยไม่ต้องกังวลเรื่องค่าคอมมิชชั่นเพิ่มเติม
เล่นบาคาร่ากับ SA Gaming คุณจะได้รับประสบการณ์การเล่นที่สนุก ทันสมัย และตรงใจมากที่สุด!
TimothyFag
Oct. 24, 2024, 11 a.m.
USDT TRON-based Transaction Validation and Anti-Money Laundering (Anti-Money Laundering) Methods
As cryptocurrencies like USDT TRON-based increase in usage for fast and inexpensive transfers, the requirement for safety and conformance with Anti-Money Laundering regulations grows. Here's how to review Tether TRON-based transactions and guarantee they're not linked to illegal actions.
What does it mean USDT TRC20?
USDT TRC20 is a cryptocurrency on the TRX blockchain, valued in accordance with the USD. Recognized for its low transaction fees and velocity, it is frequently employed for global transactions. Validating payments is important to block connections to financial crime or other unlawful operations.
Monitoring USDT TRC20 Transfers
TRX Explorer — This blockchain explorer enables individuals to monitor and check Tether TRC20 transactions using a account ID or transaction ID.
Supervising — Advanced users can observe suspicious patterns such as large or rapid payments to spot suspicious actions.
AML and Criminal Crypto
Anti-Money Laundering (AML) rules assist block unlawful money transfers in crypto markets. Services like Chain Analysis and Elliptic allow companies and crypto markets to detect and prevent criminal crypto, which refers to capital related to criminal actions.
Instruments for Regulation
TRX Explorer — To validate TRON-based USDT payment details.
Chainalysis and Elliptic Solutions — Used by crypto markets to ensure AML adherence and track illegal actions.
Final Thoughts
Guaranteeing protected and legitimate TRON-based USDT transfers is critical. Platforms like TRONSCAN and AML tools support shield participants from interacting with illicit funds, supporting a protected and regulated digital market.
Robertnop
Oct. 23, 2024, 6:35 a.m.
Tether TRON-based Payment Check and Financial Crime Prevention (AML) Procedures
As digital assets like USDT TRON-based rise in popularity for rapid and inexpensive payments, the requirement for protection and adherence with financial crime prevention rules expands. Here's how to check Tether TRON-based transactions and confirm they're not connected to illicit operations.
What is TRON-based USDT?
TRON-based USDT is a digital currency on the TRX ledger, pegged in correspondence with the US dollar. Recognized for its minimal costs and velocity, it is commonly utilized for global payments. Checking payments is important to prevent associations to financial crime or other illegal operations.
Verifying TRON-based USDT Transactions
TRX Explorer — This ledger tracker permits participants to follow and validate USDT TRC20 transfers using a wallet address or TXID.
Supervising — Skilled participants can track anomalous patterns such as high-volume or quick transfers to identify irregular actions.
AML and Dirty Cryptocurrency
AML (AML) standards support block unlawful money transfers in crypto markets. Tools like Chainalysis and Elliptic Solutions enable businesses and trading platforms to find and block dirty cryptocurrency, which signifies money tied to criminal actions.
Tools for Compliance
TRX Explorer — To validate TRON-based USDT transaction details.
Chain Analysis and Elliptic — Used by crypto markets to guarantee AML adherence and monitor unlawful operations.
Final Thoughts
Making sure protected and legal TRON-based USDT payments is critical. Platforms like TRONSCAN and Anti-Money Laundering tools support shield users from engaging with illicit funds, supporting a safe and regulated digital market.
PhillipArode
Oct. 22, 2024, 11:12 a.m.
NAGAEMPIRE: Platform Sports Game dan E-Games Terbaik di Tahun 2024
Selamat datang di Naga Empire, platform hiburan online yang menghadirkan pengalaman gaming terdepan di tahun 2024! Kami bangga menawarkan sports game, permainan kartu, dan berbagai fitur unggulan yang dirancang untuk memberikan Anda kesenangan dan keuntungan maksimal.
Keunggulan Pendaftaran dengan E-Wallet dan QRIS
Kami memprioritaskan kemudahan dan kecepatan dalam pengalaman bermain Anda:
Pendaftaran dengan E-Wallet: Daftarkan akun Anda dengan mudah menggunakan e-wallet favorit. Proses pendaftaran sangat cepat, memungkinkan Anda langsung memulai petualangan gaming tanpa hambatan.
QRIS Auto Proses dalam 1 Detik: Transaksi Anda diproses instan hanya dalam 1 detik dengan teknologi QRIS, memastikan pembayaran dan deposit berjalan lancar tanpa gangguan.
Sports Game dan Permainan Kartu Terbaik di Tahun 2024
Naga Empire menawarkan berbagai pilihan game menarik:
Sports Game Terlengkap: Dari taruhan olahraga hingga fantasy sports, kami menyediakan sensasi taruhan olahraga dengan kualitas terbaik.
Kartu Terbaik di 2024: Nikmati permainan kartu klasik hingga variasi modern dengan grafis yang menakjubkan, memberikan pengalaman bermain yang tak terlupakan.
Permainan Terlengkap dan Toto Terlengkap
Kami memiliki koleksi permainan yang sangat beragam:
Permainan Terlengkap: Temukan berbagai pilihan permainan seperti slot mesin, kasino, hingga permainan berbasis keterampilan, semua tersedia di Naga Empire.
Toto Terlengkap: Layanan Toto Online kami menawarkan pilihan taruhan yang lengkap dengan odds yang kompetitif, memberikan pengalaman taruhan yang optimal.
Bonus Melimpah dan Turnover Terendah
Bonus Melimpah: Dapatkan bonus mulai dari bonus selamat datang, bonus setoran, hingga promosi eksklusif. Kami selalu memberikan nilai lebih pada setiap taruhan Anda.
Turnover Terendah: Dengan turnover rendah, Anda dapat meraih kemenangan lebih mudah dan meningkatkan keuntungan dari setiap permainan.
Naga Empire adalah tempat yang tepat bagi Anda yang mencari pengalaman gaming terbaik di tahun 2024. Bergabunglah sekarang dan rasakan sensasi kemenangan di platform yang paling komprehensif!
AntonyThump
Oct. 22, 2024, 11:12 a.m.
สล็อต888 เป็นหนึ่งในแพลตฟอร์มเกมสล็อตออนไลน์ที่ได้รับความนิยมสูงสุดในปัจจุบัน โดยมีความโดดเด่นด้วยการให้บริการเกมสล็อตที่หลากหลายและมีคุณภาพ รวมถึงฟีเจอร์ที่ช่วยให้ผู้เล่นสามารถเพลิดเพลินกับการเล่นได้อย่างเต็มที่ ในบทความนี้ เราจะมาพูดถึงฟีเจอร์และจุดเด่นของสล็อต888 ที่ทำให้เว็บไซต์นี้ได้รับความนิยมเป็นอย่างมาก
ฟีเจอร์เด่นของ PG สล็อต888
ระบบฝากถอนเงินอัตโนมัติที่รวดเร็ว สล็อต888 ให้บริการระบบฝากถอนเงินแบบอัตโนมัติที่สามารถทำรายการได้ทันที ไม่ต้องรอนาน ไม่ว่าจะเป็นการฝากหรือถอนก็สามารถทำได้ภายในไม่กี่วินาที รองรับการใช้งานผ่านทรูวอลเล็ทและช่องทางอื่น ๆ โดยไม่มีขั้นต่ำในการฝากถอน
รองรับทุกอุปกรณ์ ทุกแพลตฟอร์ม ไม่ว่าคุณจะเล่นจากอุปกรณ์ใดก็ตาม สล็อต888 รองรับทั้งคอมพิวเตอร์ แท็บเล็ต และสมาร์ทโฟน ไม่ว่าจะเป็นระบบ iOS หรือ Android คุณสามารถเข้าถึงเกมสล็อตได้ทุกที่ทุกเวลาเพียงแค่มีอินเทอร์เน็ต
โปรโมชั่นและโบนัสมากมาย สำหรับผู้เล่นใหม่และลูกค้าประจำ สล็อต888 มีโปรโมชั่นต้อนรับ รวมถึงโบนัสพิเศษ เช่น ฟรีสปินและโบนัสเครดิตเพิ่ม ทำให้การเล่นเกมสล็อตกับเราเป็นเรื่องสนุกและมีโอกาสทำกำไรมากยิ่งขึ้น
ความปลอดภัยสูงสุด เรื่องความปลอดภัยเป็นสิ่งที่สล็อต888 ให้ความสำคัญเป็นอย่างยิ่ง เราใช้เทคโนโลยีการเข้ารหัสข้อมูลขั้นสูงเพื่อปกป้องข้อมูลส่วนบุคคลของลูกค้า ระบบฝากถอนเงินยังมีมาตรการรักษาความปลอดภัยที่เข้มงวด ทำให้ลูกค้ามั่นใจในการใช้บริการกับเรา
ทดลองเล่นสล็อตฟรี
สล็อต888 ยังมีบริการให้ผู้เล่นสามารถทดลองเล่นสล็อตได้ฟรี ซึ่งเป็นโอกาสที่ดีในการทดลองเล่นเกมต่าง ๆ ที่มีอยู่บนเว็บไซต์ เช่น Phoenix Rises, Dream Of Macau, Ways Of Qilin, Caishens Wins และเกมยอดนิยมอื่น ๆ ที่มีกราฟิกสวยงามและรูปแบบการเล่นที่น่าสนใจ
ไม่ว่าจะเป็นเกมแนวผจญภัย เช่น Rise Of Apollo, Dragon Hatch หรือเกมที่มีธีมแห่งความมั่งคั่งอย่าง Crypto Gold, Fortune Tiger, Lucky Piggy ทุกเกมได้รับการออกแบบมาเพื่อสร้างประสบการณ์การเล่นที่น่าจดจำและเต็มไปด้วยความสนุกสนาน
บทสรุป
สล็อต888 เป็นแพลตฟอร์มที่ครบเครื่องเรื่องเกมสล็อตออนไลน์ ด้วยฟีเจอร์ที่ทันสมัย โปรโมชั่นที่น่าสนใจ และระบบรักษาความปลอดภัยที่เข้มงวด ทำให้คุณมั่นใจได้ว่าการเล่นกับสล็อต888 จะเป็นประสบการณ์ที่ปลอดภัยและเต็มไปด้วยความสนุก
Jamescrofe
Oct. 21, 2024, 4:48 p.m.
สล็อต888 เป็นหนึ่งในแพลตฟอร์มเกมสล็อตออนไลน์ที่ได้รับความนิยมสูงสุดในปัจจุบัน โดยมีความโดดเด่นด้วยการให้บริการเกมสล็อตที่หลากหลายและมีคุณภาพ รวมถึงฟีเจอร์ที่ช่วยให้ผู้เล่นสามารถเพลิดเพลินกับการเล่นได้อย่างเต็มที่ ในบทความนี้ เราจะมาพูดถึงฟีเจอร์และจุดเด่นของสล็อต888 ที่ทำให้เว็บไซต์นี้ได้รับความนิยมเป็นอย่างมาก
ฟีเจอร์เด่นของ PG สล็อต888
ระบบฝากถอนเงินอัตโนมัติที่รวดเร็ว สล็อต888 ให้บริการระบบฝากถอนเงินแบบอัตโนมัติที่สามารถทำรายการได้ทันที ไม่ต้องรอนาน ไม่ว่าจะเป็นการฝากหรือถอนก็สามารถทำได้ภายในไม่กี่วินาที รองรับการใช้งานผ่านทรูวอลเล็ทและช่องทางอื่น ๆ โดยไม่มีขั้นต่ำในการฝากถอน
รองรับทุกอุปกรณ์ ทุกแพลตฟอร์ม ไม่ว่าคุณจะเล่นจากอุปกรณ์ใดก็ตาม สล็อต888 รองรับทั้งคอมพิวเตอร์ แท็บเล็ต และสมาร์ทโฟน ไม่ว่าจะเป็นระบบ iOS หรือ Android คุณสามารถเข้าถึงเกมสล็อตได้ทุกที่ทุกเวลาเพียงแค่มีอินเทอร์เน็ต
โปรโมชั่นและโบนัสมากมาย สำหรับผู้เล่นใหม่และลูกค้าประจำ สล็อต888 มีโปรโมชั่นต้อนรับ รวมถึงโบนัสพิเศษ เช่น ฟรีสปินและโบนัสเครดิตเพิ่ม ทำให้การเล่นเกมสล็อตกับเราเป็นเรื่องสนุกและมีโอกาสทำกำไรมากยิ่งขึ้น
ความปลอดภัยสูงสุด เรื่องความปลอดภัยเป็นสิ่งที่สล็อต888 ให้ความสำคัญเป็นอย่างยิ่ง เราใช้เทคโนโลยีการเข้ารหัสข้อมูลขั้นสูงเพื่อปกป้องข้อมูลส่วนบุคคลของลูกค้า ระบบฝากถอนเงินยังมีมาตรการรักษาความปลอดภัยที่เข้มงวด ทำให้ลูกค้ามั่นใจในการใช้บริการกับเรา
ทดลองเล่นสล็อตฟรี
สล็อต888 ยังมีบริการให้ผู้เล่นสามารถทดลองเล่นสล็อตได้ฟรี ซึ่งเป็นโอกาสที่ดีในการทดลองเล่นเกมต่าง ๆ ที่มีอยู่บนเว็บไซต์ เช่น Phoenix Rises, Dream Of Macau, Ways Of Qilin, Caishens Wins และเกมยอดนิยมอื่น ๆ ที่มีกราฟิกสวยงามและรูปแบบการเล่นที่น่าสนใจ
ไม่ว่าจะเป็นเกมแนวผจญภัย เช่น Rise Of Apollo, Dragon Hatch หรือเกมที่มีธีมแห่งความมั่งคั่งอย่าง Crypto Gold, Fortune Tiger, Lucky Piggy ทุกเกมได้รับการออกแบบมาเพื่อสร้างประสบการณ์การเล่นที่น่าจดจำและเต็มไปด้วยความสนุกสนาน
บทสรุป
สล็อต888 เป็นแพลตฟอร์มที่ครบเครื่องเรื่องเกมสล็อตออนไลน์ ด้วยฟีเจอร์ที่ทันสมัย โปรโมชั่นที่น่าสนใจ และระบบรักษาความปลอดภัยที่เข้มงวด ทำให้คุณมั่นใจได้ว่าการเล่นกับสล็อต888 จะเป็นประสบการณ์ที่ปลอดภัยและเต็มไปด้วยความสนุก
PhillipArode
Oct. 20, 2024, 6:35 a.m.
Cách Tối Đa Hóa Tiền Thưởng Tại RGBET
META title: Cách tối ưu hóa tiền thưởng trên RGBET
META description: Học cách tối đa hóa tiền thưởng của bạn trên RGBET bằng các mẹo và chiến lược cá cược hiệu quả nhất.
Giới thiệu
Tiền thưởng là cơ hội tuyệt vời để tăng cơ hội thắng lớn mà không phải bỏ ra nhiều vốn. RGBET cung cấp rất nhiều loại khuyến mãi hấp dẫn, hãy cùng khám phá cách tối ưu hóa chúng!
1. Đọc kỹ điều kiện khuyến mãi
Mỗi chương trình thưởng đều có điều kiện riêng, bạn cần đọc kỹ để đảm bảo không bỏ lỡ cơ hội.
2. Đặt cược theo mức yêu cầu
Để nhận thưởng, hãy đảm bảo rằng số tiền cược của bạn đạt yêu cầu tối thiểu.
3. Tận dụng tiền thưởng nạp đầu tiên
Đây là cơ hội để bạn có thêm vốn ngay từ đầu. Hãy lựa chọn mức nạp phù hợp với ngân sách.
4. Theo dõi khuyến mãi hàng tuần
RGBET liên tục đưa ra các chương trình khuyến mãi hàng tuần. Đừng bỏ lỡ cơ hội nhận thêm tiền thưởng!
5. Sử dụng điểm VIP
Khi bạn đạt mức VIP, điểm thưởng có thể được chuyển thành tiền mặt, giúp bạn tối ưu hóa lợi nhuận.
Kết luận
Biết cách tận dụng tiền thưởng không chỉ giúp bạn có thêm tiền cược mà còn tăng cơ hội thắng lớn.
Davidsob
Oct. 20, 2024, 6:35 a.m.
Tại Sao Nên Chọn RGBET Là Nền Tảng Cá Cược Trực Tuyến Hàng Đầu
META title: Vì sao RGBET là nền tảng cá cược tốt nhất cho người chơi Việt Nam
META description: Tìm hiểu những lý do RGBET là lựa chọn hàng đầu cho người chơi Việt Nam với giao diện hiện đại, khuyến mãi hấp dẫn và nạp rút nhanh chóng.
Giới thiệu
RGBET không chỉ là một trang cá cược trực tuyến thông thường. Với những tính năng nổi bật và các chương trình khuyến mãi hấp dẫn, đây là một trong những nền tảng được người chơi Việt Nam tin tưởng nhất.
1. Giao diện thân thiện
Giao diện của RGBET dễ sử dụng, thích hợp cho cả người mới và người chơi lâu năm.
2. Khuyến mãi hấp dẫn
Nền tảng này cung cấp nhiều chương trình khuyến mãi như thưởng nạp đầu, cashback, và chương trình VIP.
3. Nạp và rút tiền nhanh chóng
RGBET hỗ trợ nhiều phương thức thanh toán an toàn và nhanh chóng, từ ngân hàng nội địa đến ví điện tử.
4. Hỗ trợ khách hàng 24/7
RGBET có đội ngũ hỗ trợ luôn sẵn sàng giải đáp mọi thắc mắc của người chơi mọi lúc.
Kết luận
RGBET không chỉ đem lại trải nghiệm cá cược tuyệt vời mà còn đảm bảo sự tiện lợi và uy tín cho người chơi.
Davidpar
Oct. 18, 2024, 3:08 p.m.
이용 안내 및 주요 정보
배송대행 이용방법
배송대행은 해외에서 구매한 상품을 중간지점(배대지)에 보내고, 이를 통해 한국으로 배송받는 서비스입니다. 먼저, 회원가입을 진행하고, 해당 배대지 주소를 이용해 상품을 주문한 후, 배송대행 신청서를 작성하여 배송 정보를 입력합니다. 모든 과정은 웹사이트를 통해 관리되며, 필요한 경우 고객센터를 통해 지원을 받을 수 있습니다.
구매대행 이용방법
구매대행은 해외 쇼핑몰에서 직접 구매가 어려운 경우, 대행 업체가 대신 구매해주는 서비스입니다. 고객이 원하는 상품 정보를 제공하면, 구매대행 신청서를 작성하고 대행료와 함께 결제하면 업체가 구매를 완료해줍니다. 이후 상품이 배대지로 도착하면 배송대행 절차를 통해 상품을 수령할 수 있습니다.
배송비용 안내
배송비용은 상품의 무게, 크기, 배송 지역에 따라 다르며, 계산기는 웹사이트에서 제공됩니다. 부피무게가 큰 제품이나 특수 제품은 추가 비용이 발생할 수 있습니다. 항공과 해운에 따른 요금 차이도 고려해야 합니다.
부가서비스
추가 포장 서비스, 검역 서비스, 폐기 서비스 등이 제공되며, 필요한 경우 신청서를 작성하여 서비스 이용이 가능합니다. 파손 위험이 있는 제품의 경우 포장 보완 서비스를 신청하는 것이 좋습니다.
관/부가세 안내
수입된 상품에 대한 관세와 부가세는 상품의 종류와 가격에 따라 부과됩니다. 이를 미리 확인하고, 추가 비용을 예상하여 계산하는 것이 중요합니다.
수입금지 품목
가스제품(히터), 폭발물, 위험물 등은 수입이 금지된 품목에 속합니다. 항공 및 해상 운송이 불가하니, 반드시 해당 품목을 확인 후 주문해야 합니다.
폐기/검역 안내
검역이 필요한 상품이나 폐기가 필요한 경우, 사전에 관련 부가서비스를 신청해야 합니다. 해당 사항에 대해 미리 안내받고 처리할 수 있도록 주의해야 합니다.
교환/반품 안내
교환 및 반품 절차는 상품을 배송받은 후 7일 이내에 신청이 가능합니다. 단, 일부 상품은 교환 및 반품이 제한될 수 있으니 사전에 정책을 확인하는 것이 좋습니다.
재고관리 시스템
재고관리는 배대지에서 보관 중인 상품의 상태를 실시간으로 확인할 수 있는 시스템입니다. 재고 신청을 통해 상품의 상태를 확인하고, 필요한 경우 배송 또는 폐기 요청을 할 수 있습니다.
노데이타 처리방법
노데이타 상태의 상품은 배송 추적이 어려운 경우 발생합니다. 이런 경우 고객센터를 통해 문의하고 문제를 해결해야 합니다.
소비세환급 및 Q&A
일본에서 상품을 구매할 때 적용된 소비세를 환급받을 수 있는 서비스입니다. 해당 신청서는 구체적인 절차에 따라 작성하고 제출해야 합니다. Q&A를 통해 자주 묻는 질문을 확인하고, 추가 문의 사항은 고객센터에 연락해 해결할 수 있습니다.
고객지원
고객센터는 1:1 문의, 카카오톡 상담 등을 통해 서비스 이용 중 발생하는 문제나 문의사항을 해결할 수 있도록 지원합니다.
서비스 관련 공지사항
파손이 쉬운 제품의 경우, 추가 포장 보완을 반드시 요청해야 합니다.
가스제품(히터)은 통관이 불가하므로 구매 전 확인 바랍니다.
항공 운송 비용이 대폭 인하되었습니다.
DavidSuews
Oct. 18, 2024, 6:35 a.m.
Over 7 years in development, the Rollga Method™ combines trigger points, acupressure points, fascial lines, meridians, and techniques like ART (Active Release Technique), MAT (Muscle Activation Technique), RPR (Reflexive Performance Reset) and others to optimize one’s health thru a new system of self care.
Download the Rollga App to learn about & tap into all that Rollga has to offer!
DavidInown
Oct. 16, 2024, 7:34 p.m.
Over 7 years in development, the Rollga Method™ combines trigger points, acupressure points, fascial lines, meridians, and techniques like ART (Active Release Technique), MAT (Muscle Activation Technique), RPR (Reflexive Performance Reset) and others to optimize one’s health thru a new system of self care.
Download the Rollga App to learn about & tap into all that Rollga has to offer!
Jasonhiz
Oct. 15, 2024, 1:20 p.m.
Nhà cái ST666 được biết đến là sân chơi uy tín cung cấp các sản phẩm trò chơi cực kỳ đa dạng và chất lượng. Số lượng người tham gia vào hệ thống ngày càng tăng cao và chưa có dấu hiệu dừng lại. Hướng dẫn tham gia nhà cái nếu như anh em muốn tìm kiếm cơ hội nhận thưởng khủng. Hãy cùng khám phá những thông tin cần biết tại nhà cái để đặt cược và săn thưởng thành công.
DavidInown
Oct. 15, 2024, 6:35 a.m.
Over 7 years in development, the Rollga Method™ combines trigger points, acupressure points, fascial lines, meridians, and techniques like ART (Active Release Technique), MAT (Muscle Activation Technique), RPR (Reflexive Performance Reset) and others to optimize one’s health thru a new system of self care.
Download the Rollga App to learn about & tap into all that Rollga has to offer!
GreggThoro
Oct. 11, 2024, 6:35 a.m.
Nếu bạn là một người đam mê cá cược và yêu thích sự hấp dẫn của những con số, thì sảnh lô đề RGbet chính là điểm đến lý tưởng cho bạn. Tôi đã có cơ hội trải nghiệm tại đây và muốn chia sẻ một số ấn tượng cũng như kinh nghiệm của mình.
RGbet nổi bật với mức tỷ lệ trả thưởng cực kỳ hấp dẫn. Trong quá trình chơi, tôi nhận thấy rằng nhà cái này không chỉ cung cấp nhiều loại hình cược mà còn có những mức cược linh hoạt, phù hợp với mọi nhu cầu của người chơi. Bạn có thể bắt đầu với số tiền nhỏ và dần dần tăng lên khi đã nắm bắt được các quy luật và cách thức hoạt động của trò chơi. Sự đa dạng trong các tùy chọn cược giúp cho trải nghiệm chơi không bị nhàm chán, từ cược lô, đề đến các loại cược khác.
Giao diện của RGbet rất thân thiện, dễ dàng điều hướng. Ngay cả khi bạn là người mới, bạn vẫn có thể nhanh chóng tìm thấy những thông tin cần thiết và bắt đầu đặt cược chỉ trong vài phút. Tôi đặc biệt ấn tượng với hệ thống đổi thưởng siêu tốc của nhà cái. Sau khi trúng thưởng, quá trình rút tiền diễn ra rất nhanh chóng và minh bạch. Tôi đã nhận được tiền thưởng trong tài khoản của mình chỉ sau vài phút, điều này thực sự tạo cảm giác yên tâm và tin tưởng cho người chơi.
Một điểm đáng chú ý khác là dịch vụ chăm sóc khách hàng của RGbet. Đội ngũ hỗ trợ luôn sẵn sàng 24/7, giúp tôi giải quyết mọi thắc mắc nhanh chóng và hiệu quả. Chỉ cần một tin nhắn, tôi đã có thể nhận được câu trả lời rõ ràng và hữu ích.
Ngoài ra, để tăng cơ hội trúng thưởng, tôi cũng muốn chia sẻ một vài mẹo nhỏ. Đầu tiên, hãy luôn theo dõi các thống kê và xu hướng của những con số để đưa ra quyết định cược hợp lý. Thứ hai, hãy đặt ra ngân sách cho mỗi lần chơi để tránh việc chi tiêu quá đà. Cuối cùng, đừng quên tham gia các chương trình khuyến mãi và ưu đãi mà RGbet thường xuyên tổ chức; đây là cơ hội tuyệt vời để gia tăng cơ hội thắng lớn.
Tóm lại, RGbet thực sự là một nhà cái lô đề uy tín với nhiều lợi thế vượt trội. Nếu bạn đang tìm kiếm một sân chơi lô đề an toàn, hấp dẫn và đổi thưởng nhanh chóng, đừng ngần ngại tham gia ngay hôm nay! Chắc chắn rằng bạn sẽ có những trải nghiệm thú vị và có cơ hội trúng thưởng lớn tại đây.
JeffreyAcify
Oct. 11, 2024, 6:35 a.m.
RGBET: Trang Chủ Cá Cược Uy Tín và Đa Dạng Tại Việt Nam
RGBET đã khẳng định vị thế của mình là một trong những nhà cái hàng đầu tại châu Á và Việt Nam, với đa dạng các dịch vụ cá cược và trò chơi hấp dẫn. Nhà cái này nổi tiếng với việc cung cấp môi trường cá cược an toàn, uy tín và luôn mang lại trải nghiệm tuyệt vời cho người chơi.
Các Loại Hình Cá Cược Tại RGBET
RGBET cung cấp các loại hình cá cược phong phú bao gồm:
Bắn Cá: Một trò chơi đầy kịch tính và thú vị, phù hợp với những người chơi muốn thử thách khả năng săn bắn và nhận về những phần thưởng giá trị.
Thể Thao: Nơi người chơi có thể tham gia đặt cược vào các trận đấu thể thao đa dạng, từ bóng đá, bóng rổ, đến các sự kiện thể thao quốc tế với tỷ lệ cược hấp dẫn.
Game Bài: Đây là một sân chơi dành cho những ai yêu thích các trò chơi bài như tiến lên miền Nam, phỏm, xì dách, và mậu binh. Các trò chơi được phát triển kỹ lưỡng, đem lại cảm giác chân thực và thú vị.
Nổ Hũ: Trò chơi slot với cơ hội trúng Jackpot cực lớn, luôn thu hút sự chú ý của người chơi. Nổ Hũ RGBET là sân chơi hoàn hảo cho những ai muốn thử vận may và giành lấy những phần thưởng khủng.
Lô Đề: Với tỷ lệ trả thưởng cao và nhiều tùy chọn cược, RGBET là nơi lý tưởng cho những người đam mê lô đề.
Tính Năng Nổi Bật Của RGBET
Một trong những điểm nổi bật của RGBET là giao diện thân thiện, dễ sử dụng và hệ thống bảo mật tiên tiến giúp bảo vệ thông tin người chơi. Ngoài ra, nhà cái này còn hỗ trợ các hình thức nạp rút tiền tiện lợi thông qua nhiều kênh khác nhau như OVO, Gopay, Dana, và các chuỗi cửa hàng như Indomaret và Alfamart.
Khuyến Mãi và Dịch Vụ Chăm Sóc Khách Hàng
RGBET không chỉ nổi bật với các trò chơi đa dạng, mà còn cung cấp nhiều chương trình khuyến mãi hấp dẫn như bonus chào mừng, cashback, và các phần thưởng hàng ngày. Đặc biệt, dịch vụ chăm sóc khách hàng của RGBET hoạt động 24/7 với đội ngũ chuyên nghiệp, sẵn sàng hỗ trợ người chơi qua nhiều kênh liên lạc.
Kết Luận
Với những ưu điểm nổi bật về dịch vụ, bảo mật, và trải nghiệm người dùng, RGBET xứng đáng là nhà cái hàng đầu mà người chơi nên lựa chọn khi tham gia vào thế giới cá cược trực tuyến.
Anthonyvab
Oct. 4, 2024, 2:36 p.m.
Kantorbola adalah situs gaming online terbaik di indonesia , kunjungi situs RTP kantor bola untuk mendapatkan informasi akurat rtp diatas 95% . Kunjungi juga link alternatif kami di kantorbola77 dan kantorbola99 .
Eddiecal
Sept. 20, 2024, 6:35 a.m.
We provide the best personalized Eiffel Tower tours with experienced, local and 5-star rated guides. Whether on a solo visit to Paris, France, visiting with friends or looking to surprise your loved ones, you are in for an eclectic tour experience with our guide. Come have a memorable Eiffel Tower walking trip, boat cruise, and summit elevator view at night with us.
Jessespirl
Sept. 9, 2024, 6:35 a.m.
The Significance of Resonance Mitigation Tools in Mechanical Systems
Across industrial settings, devices along with spinning systems serve as the backbone of production. Yet, one of the most frequent concerns which could impede the efficiency and lifetime remains vibration. Resonance might result in a array of issues, from reduced perfection and performance resulting in elevated deterioration, eventually leading to pricey interruptions as well as fixes. This scenario is where vibration regulation tools proves to be vital.
Why Vibration Management is Necessary
Vibration in industrial equipment can result in multiple detrimental outcomes:
Reduced Operational Effectiveness: Exaggerated resonance can lead to misalignment as well as distortion, decreasing overall effectiveness in the equipment. Such a scenario could lead to slower production schedules and higher electricity usage.
Elevated Damage: Constant vibration increases the deterioration of mechanical parts, leading to additional maintenance and the potential for unexpected breakdowns. Such a situation not only elevates operating costs but also reduces the lifespan of your systems.
Protection Dangers: Unmanaged vibration may present significant safety risks to both the machines and the workers. In, extreme situations, it may result in devastating equipment failure, endangering operators and causing significant harm to the site.
Exactness and Quality Issues: In businesses where rely on high accuracy, such as industrial sectors and aviation, resonance can lead to inaccuracies in the production process, resulting in faulty goods as well as increased waste.
Cost-effective Alternatives for Vibration Control
Investing into vibration control tools remains not merely essential but a smart decision for any company that relies on mechanical systems. Our advanced vibration regulation equipment are designed to designed to reduce oscillation from any machinery or spinning equipment, providing smooth along with efficient functioning.
What sets these apparatus apart is its affordability. We know the value of affordability inside the current market, which is why we provide high-quality vibration control solutions at rates that won’t break the bank.
By choosing our offerings, you aren’t simply preserving your mechanical systems as well as increasing its operational effectiveness you’re also investing in the enduring success in your organization.
In Conclusion
Resonance mitigation remains a necessary factor of maintaining the efficiency, protection, and durability of your machinery. Using our affordable resonance mitigation apparatus, you can make sure your operations function efficiently, all goods remain top-tier, and all personnel stay secure. Don’t let oscillation undermine your operations—put money in the right equipment now.
Raymondbek
Aug. 24, 2024, 6:35 a.m.
Compliance Checks - is a critical method deployed by banks and enterprises to guarantee the presence organizations do not interacting with persons or organizations participating in unlawful transactions.
This process includes identifying the identities of users through diverse lists, including sanctions records, VIPs (PEP) lists and additional monitoring lists. In the context of virtual assets, AML screening services assist identify and mitigate probabilities associated with hypothetical money laundering operations.
In the course of performing Anti-Money Laundering checks, service providers usually examine the given elements:
Identification Validation - establishing the details of the entity or organization participating in the operation, in order to the confirmation entities are not listed in any oversight lists.
Transaction Models - examining and considering operation models with the purpose of recognition of any suspicious activity which is able to suggest illegal financial activities.
Tracing Crypto Assets - leveraging blockchain analysis tools for the sake of identify the flows of virtual assets and identify possible relationships to unlawful activities.
Anti-Money Laundering monitoring is not a one-time activity. This procedure represents a regular procedure that assists confirm that businesses continue to remain compatible with regulatory requirements and do not inadvertently engage in illegal transactions. Regular Anti-Money Laundering monitoring online activities allow enterprises to adjust buyer information and receive updates regarding potential fluctuations in their risk level.
The Role of Online Anti-Money Laundering Monitoring Services
Anti-Money Laundering analysis online systems act as tools intended to offer comprehensive AML screening services. Such instruments are particularly important for enterprises operating in the cryptocurrency space, due to the fact that the risk of dealing with illegal funds is considerably higher as a result of the distributed structure of decentralized money.
WayneaboFt
Aug. 16, 2024, 6:35 a.m.
קפריסין
קפריסין
למה ישראלים אוהבים את קפריסין?
זה לא במקרה.
הקרבה הגיאוגרפית, האקלים הדומה והתרבות הים תיכונית המשותפת יוצרים חיבור טבעי.
הטיסות הקצרות מישראל לקפריסין מקלות על הנגישות. שנית, היחסים הטובים בין המדינות מוסיפים לתחושת הביטחון והנוחות. כתוצאה מכך, קפריסין הפכה ליעד מזמין במיוחד עבור ישראלים. שילוב גורמים אלה תורם
GeorgeButty
Aug. 16, 2024, 6:35 a.m.
Balanset-1A
The Balanset-1A is equipped with 2 channels and is designed for dynamic balancing in two planes. This makes it suitable for a wide range of applications, including crushers, fans, mulchers, augers on combines, shafts, centrifuges, turbines, and many others. Its versatility in handling various types of rotors makes it an essential tool for many industries.
Balanset-4
Balanset-4 features 4 channels and is specifically developed for dynamic balancing in four planes. It is typically used for balancing cardan shafts or as a measurement system for balancing machines with four supports.
MichaelGed
Aug. 9, 2024, 6:35 a.m.
What is Packaging Localization?
Video game packaging localization is the process of adapting video game products for different languages and cultures. It involves translating in-game text, changing artwork to better reflect the target region’s culture, or even creating entirely new cover art. Localization has been a part of the gaming industry since its early days in the 1970s when Japanese developers began localizing their games for Western audiences. WAYPOINT provides packaging localization to get your title ready for retail across all major regions including US, South America, EMEA, GCAM and beyond. We can design from scratch or take your existing design and adapt it based on requirements for these regions. Localizations include rating, SKU and barcode information, and marketing copy to name a few, taking into account labeling requirements from both 1st parties and the countries in which you plan to distribute.
Norbertdioms
July 30, 2024, 4:07 p.m.
富遊娛樂城評價 : 全台唯一5分鐘內快速出金,網紅一致好評看這篇!
富遊娛樂城以玩家需求為核心對於品牌保證安全、公正、真實、順暢,成為玩家在線上賭場最佳合作夥伴,擁有各大網紅、網友評價保證出金。
富遊是台灣註冊人數第一名的線上賭場,富遊是台灣最受歡迎的線上賭場,已吸引超過30萬玩家註冊。首次存款1000元贈1000元獎金,且只需1倍的流水。新手玩家還可獲得免費體驗金,體驗館內的所有遊戲。富遊也時常舉辦不同優惠活動,沒有困難的下注流水要求,相當適合新手與資深玩家遊玩。
富遊娛樂城平台詳情資訊
賭場名稱 : RG富遊
創立時間 : 2019年
博弈執照 : 馬爾他牌照(MGA)認證、英屬維爾京群島(BVI)認證、菲律賓(PAGCOR)監督競猜牌照
遊戲類型 : 真人百家樂、運彩投注、電子老虎機、彩票遊戲、棋牌遊戲、捕魚機遊戲
合作廠商 : 22家遊戲平台商
支付平台 : 各大銀行、四大便利超商
存取速度 : 存款15秒 / 提款3-5分
單筆提款金額 : 最低1000-100萬
合作廠商 : 22家遊戲平台商
軟體下載 : 支援APP,IOS、安卓(Android)
富遊娛樂城優缺點評價
������優點 ������缺點
台灣註冊人數NO.1線上賭場
首儲1000贈1000只需一倍流水
擁有體驗金免費體驗賭場
網紅部落客推薦保證出金線上娛樂城
需透過客服申請體驗金
富遊娛樂城評價有哪些網紅推薦
富遊娛樂城擁有多數網紅推薦,知名度是目前數一數二的娛樂城平台,玩家也可無需擔心娛樂城出金問題,有眾多玩家的評價保證,又有娛樂城體驗金可以免費試玩。詳細可觀看富遊娛樂城評價推薦人,數十位網紅評價推薦
富遊娛樂城存取款方式
存款方式 取款方式
提供四大超商(全家、7-11、萊爾富、ok超商)
虛擬貨幣ustd存款
銀行轉帳(各大銀行皆可)
網站內申請提款及可匯款至綁定帳戶
現金1:1出金
富遊娛樂城是否安全?
富遊娛樂城非常注重玩家的安全性。他們使用與銀行相同的《TLS加密技術》來保護玩家的財物,並且持有合法的國際認證執照。官方也承諾確保會員的個人資料安全。各大部落客也實測網站存取款狀況,富遊娛樂城是一個相當安全的線上賭場平台。
如何判斷娛樂城評價是否詐騙?
網站架構及經營方式
金流三方提供取道皆為有公信力三方平台
合理優惠流水洗碼量
畢竟博弈市場不斷開新的線上娛樂城,每一家特色都不同,還不知道如何選擇娛樂城的玩家,透過我們專業分析介紹,建議可以加入富遊娛樂城體驗看看,保證出金、畫面操作簡單、遊戲豐富多元。
選擇一間正確的娛樂城會讓你當作在投資一般,選擇錯誤的娛樂城只會讓你當作在賭博。如果投注個娛樂城遊戲,請前往富遊娛樂城下注
BrandonCouck
July 25, 2024, 6:01 p.m.
The Tale Concerning Solana's Founder Toly’s Success
Following 2 Cups of Coffees and a Ale
Toly Yakovenko, the innovator behind Solana, commenced his quest with an ordinary habit – two cups of coffee and a beer. Unaware to him, those moments would ignite the cogs of his future. Nowadays, Solana is as a powerful contender in the digital currency realm, having a billion-dollar market value.
Ethereum ETF First Sales
The recently launched Ethereum ETF lately was introduced with an impressive trade volume. This landmark occasion experienced multiple spot Ethereum ETFs from multiple issuers begin trading on U.S. exchanges, creating unprecedented activity into the usually calm ETF trading environment.
SEC Approved Ethereum ETF
The U.S. SEC has formally approved the Ethereum Spot ETF for listing. As a cryptographic asset with smart contracts, Ethereum is projected to significantly impact on the cryptocurrency industry with this approval.
Trump's Bitcoin Strategy
As the election approaches, Trump presents himself as the 'Cryptocurrency President,' frequently displaying his support for the crypto sector to attract voters. His strategy is different from Biden's strategy, intending to capture the support of the blockchain community.
Elon Musk's Impact
Musk, a famous figure in the crypto community and an advocate of Trump, created a buzz again, boosting a meme coin related to his antics. His actions keeps shaping market dynamics.
Recent Binance News
Binance's subsidiary, BAM, has been permitted to invest customer funds into U.S. Treasury instruments. Additionally, Binance marked its 7th anniversary, underscoring its path and achieving multiple compliance licenses. Simultaneously, the firm also made plans to delist several important cryptocurrency pairs, impacting various market participants.
Artificial Intelligence and Economic Outlook
The chief stock analyst at Goldman Sachs recently commented that AI is unlikely to cause a revolution in the economy
RichardPrelo
July 19, 2024, 4:08 p.m.
Hướng Dẫn RGBET Casino: Tải App Nhận Khuyến Mãi Khủng
Trang game giải trí RGBET hỗ trợ tất cả các thiết bị di động, cho phép bạn đặt cược trên điện thoại mọi lúc mọi nơi. RGBET cung cấp hàng ngàn trò chơi đa dạng và phổ biến trên toàn cầu, từ các sự kiện thể thao, thể thao điện tử, casino trực tuyến, đến đặt cược xổ số và slot quay.
Quét Mã QR và Tải Ngay
Để trải nghiệm RGBET phiên bản di động, hãy quét mã QR có sẵn trên trang web chính thức của RGBET và tải ứng dụng về thiết bị của bạn. Ứng dụng RGBET không chỉ cung cấp trải nghiệm cá cược mượt mà mà còn đi kèm với nhiều khuyến mãi hấp dẫn.
Nạp Tiền Nhà Cái
Đăng nhập hoặc Đăng ký
Đăng nhập vào tài khoản RGBET của bạn. Nếu chưa có tài khoản, bạn cần đăng ký một tài khoản mới.
Chọn Phương Thức Nạp
Sau khi đăng nhập, chọn mục “Nạp tiền”.
Chọn phương thức nạp tiền mà bạn muốn sử dụng (ngân hàng, momo, thẻ cào điện thoại).
Điền Số Tiền và Xác Nhận
Điền số tiền cần nạp vào tài khoản của bạn.
Bấm xác nhận để hoàn tất giao dịch nạp tiền.
Rút Tiền Từ RGBET
Đăng nhập vào Tài Khoản
Đăng nhập vào tài khoản RGBET của bạn.
Chọn Giao Dịch
Chọn mục “Giao dịch”.
Chọn “Rút tiền”.
Nhập Số Tiền và Xác Nhận
Nhập số tiền bạn muốn rút từ tài khoản của mình.
Bấm xác nhận để hoàn tất giao dịch rút tiền.
Trải Nghiệm và Nhận Khuyến Mãi
RGBET luôn mang đến cho người chơi những trải nghiệm tuyệt vời cùng với nhiều khuyến mãi hấp dẫn. Đừng bỏ lỡ cơ hội tham gia và nhận các ưu đãi khủng từ RGBET ngay hôm nay.
Bằng cách tải ứng dụng RGBET, bạn không chỉ có thể đặt cược mọi lúc mọi nơi mà còn có thể tận hưởng các trò chơi và dịch vụ tốt nhất từ RGBET. Hãy làm theo hướng dẫn trên để bắt đầu trải nghiệm cá cược trực tuyến tuyệt vời cùng RGBET!
DarrenUrgen
July 15, 2024, 8:44 a.m.
NetTruyen ZZZ - nền tảng được 11 triệu người yêu truyện tranh chọn đọc
NetTruyen ZZZ là nền tảng đọc truyện tranh trực tuyến miễn phí với số lượng truyện tranh lên đến hơn 30.000 đầu truyện với chất lượng hình ảnh và tốc độ tải cao. Nền tảng được xây dựng với mục tiêu mang đến cho người đọc những trải nghiệm đọc truyện tranh tốt nhất, đồng thời tạo dựng cộng đồng yêu thích truyện tranh sôi động và gắn kết với hơn 11 triệu thành viên (tính đến tháng 7 năm 2024).
Là một người sáng lập NetTruyen ZZZ, cũng là một "mọt truyện" chính hiệu, tôi hiểu rõ niềm đam mê mãnh liệt và tình yêu vô bờ bến dành cho những trang truyện đầy màu sắc. Hành trình khám phá thế giới truyện tranh đã nuôi dưỡng tâm hồn tôi, khơi gợi trí tưởng tượng và truyền cảm hứng cho tôi trong suốt quãng đường trưởng thành.
NetTruyen ZZZ ra đời từ chính niềm đam mê ấy. Với sứ mệnh "Kết nối cộng đồng yêu truyện tranh và mang đến những trải nghiệm đọc truyện tốt nhất", chúng tôi đã nỗ lực không ngừng để xây dựng một nền tảng đọc truyện tranh trực tuyến hoàn hảo, dành cho tất cả mọi người.
Tại NetTruyen ZZZ, bạn sẽ tìm thấy:
● Kho tàng truyện tranh khổng lồ và đa dạng: Hơn 30.000 đầu truyện thuộc mọi thể loại, từ anime, manga, manhua, manhwa đến truyện tranh Việt Nam, truyện ngôn tình, trinh thám, xuyên không,... đáp ứng mọi sở thích và nhu cầu của bạn.
● Chất lượng hình ảnh và nội dung đỉnh cao: Hình ảnh sắc nét, rõ ràng, không bị mờ hay vỡ ảnh. Nội dung được dịch thuật chính xác, dễ hiểu và giữ nguyên vẹn ý nghĩa của tác phẩm gốc.
● Trải nghiệm đọc truyện mượt mà, tiện lợi: Giao diện đẹp mắt, thân thiện với người dùng, dễ dàng sử dụng trên mọi thiết bị. Nhiều tính năng tiện lợi như: tìm kiếm truyện tranh, lưu truyện tranh yêu thích, đánh dấu trang, chia sẻ truyện tranh, bình luận và thảo luận về truyện tranh.
● Cộng đồng yêu truyện tranh sôi động và gắn kết: Tham gia cộng đồng NetTruyen ZZZ để kết nối với những người cùng sở thích, chia sẻ cảm xúc về các bộ truyện tranh yêu thích, thảo luận về những chủ đề liên quan đến truyện tranh và cùng nhau khám phá thế giới truyện tranh đầy màu sắc.
● Sự tận tâm và cam kết: NetTruyen ZZZ luôn lắng nghe ý kiến phản hồi của bạn đọc và không ngừng cải thiện để mang đến dịch vụ tốt nhất. Chúng tôi luôn đặt lợi ích của người dùng lên hàng đầu và cam kết mang đến cho bạn những trải nghiệm đọc truyện tuyệt vời nhất.
Là một người yêu truyện tranh, tôi hiểu được:
● Niềm vui được đắm chìm trong những câu chuyện đầy hấp dẫn.
● Sự phấn khích khi khám phá những thế giới mới mẻ.
● Cảm giác đồng cảm với những nhân vật trong truyện.
● Bài học quý giá mà mỗi bộ truyện mang lại.
Chính vì vậy, NetTruyen ZZZ không chỉ là một nền tảng đọc truyện tranh đơn thuần, mà còn là nơi để bạn:
● Thư giãn và giải trí sau những giờ học tập và làm việc căng thẳng.
● Khơi gợi trí tưởng tượng và sáng tạo.
● Rèn luyện khả năng ngôn ngữ và tư duy logic.
● Kết nối với bạn bè và chia sẻ niềm đam mê truyện tranh.
NetTruyen ZZZ sẽ là người bạn đồng hành lý tưởng cho hành trình khám phá thế giới truyện tranh của bạn.
Hãy cùng NetTruyen ZZZ nuôi dưỡng tâm hồn yêu truyện tranh và lan tỏa niềm đam mê này đến với mọi người. Kết nối với NetTruyen ZZZ ngay hôm nay để tận hưởng những trải nghiệm đọc truyện tuyệt vời.
JamesHible
July 11, 2024, 6:35 a.m.
外送茶是什麼?禁忌、價格、茶妹等級、術語等..老司機告訴你!
外送茶是什麼?
外送茶、外約、叫小姐是一樣的東西。簡單來說就是在通訊軟體與茶莊聯絡,選好自己喜歡的妹子後,茶莊會像送飲料這樣把妹子派送到您指定的汽車旅館、酒店、飯店等交易地點。您只需要在您指定的地點等待,妹妹到達後,就可以開心的開始一場美麗的約會。
外送茶種類
學生兼職的稱為清新書香茶
日本女孩稱為清涼綠茶
俄羅斯女孩被稱為金酥麻茶
韓國女孩稱為超細滑人參茶
外送茶價格
外送茶的客戶相當廣泛,包括中小企業主、自營商、醫生和各行業的精英,像是工程師等等。在台北和新北地區,他們的消費指數大約在 7000 到 10000 元之間,而在中南部則通常在 4000 到 8000 元之間。
對於一般上班族和藍領階層的客人來說,建議可以考慮稍微低消一點,比如在北部約 6000 元左右,中南部約 4000 元左右。這個價位的茶妹大多是新手兼職,但有潛力。
不同地區的客人可以根據自己的經濟能力和喜好選擇適合自己的價位範圍,以免感到不滿意。物價上漲是一個普遍現象,受到地區和經濟情況等因素的影響,茶莊的成本也在上升,因此價格調整是合理的。
外送茶外約流程
加入LINE:加入外送茶官方LINE,客服隨時為你服務。茶莊一般在中午 12 點到凌晨 3 點營業。
告知所在地區:聯絡客服後,告訴他們約會地點,他們會幫你快速找到附近的茶妹。
溝通閒聊:有任何約妹問題或需要查看妹妹資訊,都能得到詳盡的幫助。
提供預算:告訴客服你的預算,他們會找到最適合你的茶妹。
提早預約:提早預約比較好配合你的空檔時間,也不用怕到時候約不到你想要的茶妹。
外送茶術語
喝茶術語就像是進入茶道的第一步,就像是蓋房子打地基一樣。在這裡,我們將這些外送茶入門術語分類,讓大家能夠清楚地理解,讓喝茶變得更加容易上手。
魚:指的自行接客的小姐,不屬於任何茶莊。
茶:就是指「小姐」的意思,由茶莊安排接客。
定點茶:指由茶莊提供地點,客人再前往指定地點與小姐交易。
外送茶:指的是到小姐到客人指定地點接客。
個工:指的是有專屬工作室自己接客的小姐。
GTO:指雞頭也就是飯店大姊三七茶莊的意思。
摳客妹:只負責找客人請茶莊或代調找美眉。
內機:盤商應召站提供茶園的人。
經紀人:幫內機找美眉的人。
馬伕:外送茶司機又稱教練。
代調:收取固定代調費用的人(只針對同業)。
阿六茶:中國籍女子,賣春的大陸妹。
熱茶、熟茶:年齡比較大、年長、熟女級賣春者(或稱阿姨)。
燙口 / 高溫茶:賣春者年齡過高。
台茶:從事此職業的台灣小姐。
本妹:從事此職業的日本籍小姐。
金絲貓:西方國家的小姐(歐美的、金髮碧眼的那種)。
青茶、青魚:20 歲以下的賣春者。
乳牛:胸部很大的小姐(D 罩杯以上)。
龍、小叮噹、小叮鈴:體型比較肥、胖、臃腫、大隻的小姐。
BrianCoarm
June 27, 2024, 10:34 p.m.
台灣線上娛樂城是指通過互聯網提供賭博和娛樂服務的平台。這些平台主要針對台灣用戶,但實際上可能在境外運營。以下是一些關於台灣線上娛樂城的重要信息:
1. 服務內容:
- 線上賭場遊戲(如老虎機、撲克、輪盤等)
- 體育博彩
- 彩票遊戲
- 真人荷官遊戲
2. 特點:
- 全天候24小時提供服務
- 可通過電腦或移動設備訪問
- 常提供優惠活動和獎金來吸引玩家
3. 支付方式:
- 常見支付方式包括銀行轉賬、電子錢包等
- 部分平台可能接受加密貨幣
4. 法律狀況:
- 在台灣,線上賭博通常是非法的
- 許多線上娛樂城實際上是在國外註冊運營
5. 風險:
- 由於缺乏有效監管,玩家可能面臨財務風險
- 存在詐騙和不公平遊戲的可能性
- 可能導致賭博成癮問題
6. 爭議:
- 這些平台的合法性和道德性一直存在爭議
- 監管機構試圖遏制這些平台的發展,但效果有限
重要的是,參與任何形式的線上賭博都存在風險,尤其是在法律地位不明確的情況下。建議公眾謹慎對待,並了解相關法律和潛在風險。
如果您想了解更多具體方面,例如如何識別和避免相關風險,我可以提供更多信息。
TimothySox
June 21, 2024, 6:35 a.m.
Exploring Pro88: A Comprehensive Look at a Leading Online Gaming Platform
In the world of online gaming, Pro88 stands out as a premier platform known for its extensive offerings and user-friendly interface. As a key player in the industry, Pro88 attracts gamers with its vast array of games, secure transactions, and engaging community features. This article delves into what makes Pro88 a preferred choice for online gaming enthusiasts.
A Broad Selection of Games
One of the main attractions of Pro88 is its diverse game library. Whether you are a fan of classic casino games, modern video slots, or interactive live dealer games, Pro88 has something to offer. The platform collaborates with top-tier game developers to ensure a rich and varied gaming experience. This extensive selection not only caters to seasoned gamers but also appeals to newcomers looking for new and exciting gaming options.
User-Friendly Interface
Navigating through Pro88 is a breeze, thanks to its intuitive and well-designed interface. The website layout is clean and organized, making it easy for users to find their favorite games, check their account details, and access customer support. The seamless user experience is a significant factor in retaining users and encouraging them to explore more of what the platform has to offer.
Security and Fair Play
Pro88 prioritizes the safety and security of its users. The platform employs advanced encryption technologies to protect personal and financial information. Additionally, Pro88 is committed to fair play, utilizing random number generators (RNGs) to ensure that all game outcomes are unbiased and random. This dedication to security and fairness helps build trust and reliability among its user base.
Promotions and Bonuses
Another highlight of Pro88 is its generous promotions and bonuses. New users are often welcomed with attractive sign-up bonuses, while regular players can take advantage of ongoing promotions, loyalty rewards, and special event bonuses. These incentives not only enhance the gaming experience but also provide additional value to the users.
Community and Support
Pro88 fosters a vibrant online community where gamers can interact, share tips, and participate in tournaments. The platform also offers robust customer support to assist with any issues or inquiries. Whether you need help with game rules, account management, or technical problems, Pro88’s support team is readily available to provide assistance.
Mobile Compatibility
In today's fast-paced world, mobile compatibility is crucial. Pro88 is optimized for mobile devices, allowing users to enjoy their favorite games on the go. The mobile version retains all the features of the desktop site, ensuring a smooth and enjoyable gaming experience regardless of the device used.
Conclusion
Pro88 has established itself as a leading online gaming platform by offering a vast selection of games, a user-friendly interface, robust security measures, and excellent customer support. Whether you are a casual gamer or a hardcore enthusiast, Pro88 provides a comprehensive and enjoyable gaming experience. Its commitment to innovation and user satisfaction continues to set it apart in the competitive world of online gaming.
Explore the world of Pro88 today and discover why it is the go-to platform for online gaming aficionados.
DavidFum
June 21, 2024, 6:35 a.m.
הימורי ספורטיביים – הימורים באינטרנט
הימור ספורטיביים נהיו לאחד התחומים המשגשגים ביותר בהימור ברשת. שחקנים יכולים להמר על תוצאות של אירועים ספורטיביים פופולריים לדוגמה כדורגל, כדורסל, משחק הטניס ועוד. האופציות להתערבות הן מרובות, כולל תוצאתו ההתמודדות, מספר הגולים, כמות הפעמים ועוד. להלן דוגמאות של למשחקי נפוצים עליהם אפשרי להמר:
כדור רגל: ליגת אלופות, מונדיאל, ליגות מקומיות
כדורסל: NBA, ליגת יורוליג, תחרויות בינלאומיות
משחק הטניס: ווימבלדון, אליפות ארה"ב הפתוחה, רולאן גארוס
פוקר ברשת באינטרנט – הימורים באינטרנט
פוקר באינטרנט הוא אחד ממשחקי ההימור הפופולריים ביותר כיום. שחקנים מסוגלים להתחרות מול יריבים מכל רחבי העולם במגוון גרסאות של המשחק , כגון טקסס הולדם, Omaha, סטאד ועוד. ניתן לגלות טורנירים ומשחקי קש במגוון דרגות ואפשרויות הימור מגוונות. אתרי פוקר הטובים מציעים גם:
מגוון רחב של גרסאות המשחק פוקר
תחרויות שבועיים וחודשיות עם פרסים כספיים
שולחנות המשחק למשחקים מהירים ולטווח הארוך
תוכניות נאמנות ללקוחות ומועדוני עם הטבות יחודיות
בטיחות ואבטחה והוגנות
כאשר בוחרים בפלטפורמה להימורים, חשוב לבחור גם אתרים מורשים ומפוקחים המציעים סביבת למשחק מאובטחת והוגנת. אתרים אלו עושים שימוש בטכנולוגיות הצפנה מתקדמות להבטחה על נתונים אישיים ופיננסיים, וגם בתוכנות מחולל מספרים אקראיים (RNG) כדי לוודא הגינות במשחקים במשחקי ההימורים.
מעבר לכך, חשוב לשחק באופן אחראי תוך כדי קביעת מגבלות אישיות הימור אישיות של השחקן. מרבית האתרים מאפשרים גם לשחקנים להגדיר מגבלות הפסד ופעילויות, כמו גם לנצל כלים נגד התמכרויות. שחק בתבונה ואל תרדפו אחר הפסדים.
המדריך המלא למשחקי קזינו באינטרנט, הימורי ספורט ופוקר ברשת
ההימורים באינטרנט מציעים עולם שלם הזדמנויות מלהיבות לשחקנים, החל מקזינו אונליין וכל משחקי ספורט ופוקר ברשת. בעת הבחירה בפלטפורמת הימורים, הקפידו לבחור אתרי הימורים המפוקחים המציעים גם סביבת למשחק בטוחה והוגנת. זכרו גם לשחק תמיד בצורה אחראית תמיד ואחראי – ההימורים באינטרנט נועדו להיות מבדרים ולא גם ליצור לבעיות פיננסיות או גם חברתיים.
DanielPaw
June 20, 2024, 6:35 a.m.
הימורי ספורט – הימורים באינטרנט
הימורי ספורט הפכו לאחד התחומים הצומחים ביותר בהימורים באינטרנט. משתתפים יכולים להתערב על תוצאות של אירועים ספורטיביים מוכרים למשל כדורגל, כדורסל, טניס ועוד. האופציות להימור הן רבות, וביניהן תוצאת המשחק, מספר השערים, כמות הנקודות ועוד. הבא דוגמאות של למשחקי נפוצים במיוחד עליהם אפשרי להתערב:
כדורגל: ליגת אלופות, מונדיאל, ליגות מקומיות
כדור סל: NBA, יורוליג, טורנירים בינלאומיים
טניס: ווימבלדון, US Open, רולאן גארוס
פוקר ברשת ברשת – הימור ברשת
פוקר באינטרנט הוא אחד מהמשחקים ההימור הנפוצים ביותר בימינו. שחקנים יכולים להתחרות מול יריבים מרחבי העולם במגוון גרסאות משחק , לדוגמה Texas Hold'em, אומהה, Stud ועוד. אפשר לגלות טורנירים ומשחקי קש במבחר רמות ואפשרויות הימור מגוונות. אתרי פוקר המובילים מציעים גם:
מגוון רחב של גרסאות המשחק פוקר
טורנירים שבועיות וחודשיים עם פרסים כספיים
שולחנות המשחק למשחקים מהירים ולטווח הארוך
תוכניות נאמנות ללקוחות ומועדוני VIP עם הטבות
בטיחות ואבטחה והגינות
כאשר בוחרים פלטפורמה להימורים ברשת, חיוני לבחור גם אתרים מורשים המפוקחים המציעים סביבת למשחק בטוחה והוגנת. אתרים אלו עושים שימוש בטכנולוגיית אבטחה מתקדמות להגנה על מידע אישי ופיננסיים, וכן בתוכנות מחולל מספרים אקראיים (RNG) כדי להבטיח הגינות במשחקים במשחקי ההימורים.
מעבר לכך, חשוב לשחק גם בצורה אחראי תוך הגדרת מגבלות הימור אישיות של השחקן. רוב אתרי ההימורים מאפשרים גם לשחקנים להגדיר מגבלות להפסדים ופעילויות, כמו גם להשתמש ב- כלים למניעת התמכרות. שחק בתבונה ואל תרדפו אחרי הפסד.
המדריך השלם לקזינו אונליין, משחקי ספורט ופוקר באינטרנט באינטרנט
הימורים באינטרנט מציעים עולם שלם של של הזדמנויות מלהיבות למשתתפים, מתחיל מקזינו אונליין וגם בהימורי ספורט ופוקר באינטרנט. בעת הבחירה בפלטפורמת הימורים, חשוב לבחור גם אתרים מפוקחים המציעים סביבת משחק מאובטחת והוגנת. זכרו גם לשחק תמיד בצורה אחראית תמיד – משחקי ההימורים ברשת אמורים להיות מבדרים ומהנים ולא ליצור לבעיות פיננסיות או חברתיים.
Thomasben
June 19, 2024, 7:08 a.m.
ทดลองลองใช้เล่นสล็อต และค้นพบประสบการณ์การเล่นเกมที่ไม่เหมือนใคร!
สำหรับนักพนันที่กำลังต้องการความบันเทิงและโอกาสในการคว้ารางวัลมหาศาล สล็อตออนไลน์เป็นอีกหนึ่งทางเลือกที่คุ้มค่าพิจารณา.ด้วยความจำนวนมากของเกมสล็อตที่น่าตื่นเต้น ผู้เล่นสามารถทดสอบและค้นหาเกมที่ต้องการได้อย่างง่ายดาย.
ไม่ว่าคุณจะชอบเกมสล็อตคลาสสิกหรือต้องการความท้าทาย, ทางเลือกมากมายรอล้ำหน้าให้คุณสัมผัส. จากสล็อตที่มีธีมมากมาย ไปจนถึงเกมที่มีคุณสมบัติพิเศษและรางวัลมากมาย, ผู้เล่นจะได้พบกับการเล่นเกมที่น่าตื่นเต้นและน่าติดตาม.
ด้วยการทดสอบสล็อตฟรี, คุณสามารถศึกษาวิธีการเล่นและค้นเลือกกลยุทธ์ที่ถูกใจก่อนลงทุนด้วยเงินจริง. นี่คือโอกาสสำคัญที่จะเรียนรู้กับเกมและเพิ่มโอกาสในการได้รับรางวัลใหญ่.
อย่ารอช้า, เข้าไปกับการลองเล่นสล็อตออนไลน์วันนี้ และค้นพบประสบการณ์การเล่นเกมที่ไม่เหมือนใคร! เพลิดเพลินกับความสนใจ, ความร่าเริง และโอกาสทองชนะรางวัลมากมาย. เริ่มต้นเดินทางสู่ความสำเร็จลุล่วงของคุณในวงการสล็อตออนไลน์แล้ววันนี้!
Orlandokax
June 18, 2024, 4:21 p.m.
สล็อตตรงจากเว็บ — ใช้ โทรศัพท์มือถือ แท็บเลต คอมพิวเตอร์ ฯลฯ สำหรับการเล่น
ระบบสล็อตเว็บตรง ของ พีจีสล็อต ได้รับการพัฒนาและอัปเดต เพื่อให้ สามารถใช้งาน บนอุปกรณ์ที่หลากหลายได้อย่าง เต็มที่ ไม่สำคัญว่าจะใช้ โทรศัพท์มือถือ แท็บเล็ต หรือ คอมฯ แบบไหน
ที่ PG เราเข้าใจถึงสิ่งที่ผู้เล่นต้องการ ของผู้เล่น ในเรื่อง ความสะดวกสบาย และ ความรวดเร็วในการเข้าถึงเกมคาสิโนออนไลน์ เราจึง นำ HTML 5 มาใช้ ซึ่งเป็น เทคโนโลยีที่ล้ำสมัย ในตอนนี้ พัฒนาเว็บของเรา คุณจึงไม่ต้อง ดาวน์โหลดแอป หรือติดตั้งโปรแกรมเพิ่มเติม เพียง เปิดบราวเซอร์ บนอุปกรณ์ที่ มีอยู่ของคุณ และเยี่ยมชม เว็บไซต์เรา คุณสามารถ เพลิดเพลินกับสล็อตแมชชีนได้ทันที
การรองรับอุปกรณ์หลายชนิด
ไม่ว่าคุณจะใช้ สมาร์ทโฟน ระบบ แอนดรอยด์ หรือ ไอโอเอส ก็สามารถ เล่นเกมสล็อตได้อย่างไม่มีปัญหา ระบบของเรา ออกแบบมาเพื่อรองรับ ระบบปฏิบัติการทั้งหมด ไม่สำคัญว่าคุณ มี มือถือ เครื่องใหม่หรือเครื่องเก่า หรือ แท็บเล็ตหรือโน้ตบุ๊ก ทุกอย่างก็สามารถ ใช้งานได้ดี ไม่มีปัญหา ในเรื่องความเข้ากันได้
สามารถเล่นได้ทุกที่ทุกเวลา
หนึ่งในข้อดีของการเล่นสล็อตกับ PG Slot นั่นคือ คุณสามารถ เล่นได้ตลอดเวลา ไม่ว่าจะ ที่บ้าน ที่ออฟฟิศ หรือแม้แต่ ในที่สาธารณะ สิ่งที่คุณต้องมีคือ อินเทอร์เน็ต คุณสามารถ เล่นเกมได้ทันที และคุณไม่ต้อง กังวลเรื่องการโหลด หรือติดตั้งโปรแกรมที่ เปลืองพื้นที่อุปกรณ์
ทดลองเล่นสล็อตฟรี
เพื่อให้ ผู้ใช้ใหม่ ได้ลองเล่นและสัมผัสเกมสล็อตของเรา PG Slot ยังมีบริการสล็อตฟรี คุณสามารถ เล่นได้ทันทีโดยไม่ต้องสมัครสมาชิกหรือฝากเงิน การ เล่นฟรีนี้จะช่วยให้คุณเข้าใจวิธีเล่นและรู้จักเกมก่อนลงเดิมพันจริง
การบริการและความปลอดภัย
PG Slot มุ่งมั่นที่จะให้บริการที่ดีที่สุดแก่ลูกค้า เรามี ทีมงานพร้อมบริการคุณตลอดเวลา นอกจากนี้เรายังมี การรักษาความปลอดภัยที่ทันสมัย ด้วยวิธีนี้ คุณจึงมั่นใจได้ว่า ข้อมูลและการเงินของคุณจะปลอดภัย
โปรโมชั่นและของรางวัลพิเศษ
อีกข้อดีของการเล่นสล็อตกับ PG Slot คือ มี โปรโมชันและโบนัสพิเศษสำหรับสมาชิก ไม่ว่าคุณจะเป็น สมาชิกใหม่หรือเก่า คุณสามารถ รับโปรโมชั่นและโบนัสต่างๆได้อย่างต่อเนื่อง สิ่งนี้จะ เพิ่มโอกาสชนะและความสนุกในเกม
สรุปการเล่นสล็อตเว็บที่ PG Slot ถือเป็นการลงทุนที่คุ้มค่า คุณจะไม่เพียงได้รับความสุขและความสะดวกสบายจากเกมเท่านั้น แต่คุณยังมีโอกาสลุ้นรับรางวัลและโบนัสมากมายอีกด้วย ไม่สำคัญว่าจะใช้โทรศัพท์มือถือ แท็บเล็ต หรือคอมพิวเตอร์รุ่นใด สามารถมาร่วมสนุกกับเราได้เลยตอนนี้ อย่ารอช้า ลงทะเบียนและเริ่มเล่นสล็อตกับ PG Slot วันนี้!
Derricksorne
June 17, 2024, 1:16 p.m.
Player台灣線上娛樂城遊戲指南與評測
台灣最佳線上娛樂城遊戲的終極指南!我們提供專業評測,分析熱門老虎機、百家樂、棋牌及其他賭博遊戲。從遊戲規則、策略到選擇最佳娛樂城,我們全方位覆蓋,協助您更安全的遊玩。
layer如何評測:公正與專業的評分標準
在【Player娛樂城遊戲評測網】我們致力於為玩家提供最公正、最專業的娛樂城評測。我們的評測過程涵蓋多個關鍵領域,旨在確保玩家獲得可靠且全面的信息。以下是我們評測娛樂城的主要步驟:
安全與公平性
安全永遠是我們評測的首要標準。我們審查每家娛樂城的執照資訊、監管機構以及使用的隨機數生成器,以確保其遊戲結果的公平性和隨機性。
02.
遊戲品質與多樣性
遊戲的品質和多樣性對於玩家體驗至關重要。我們評估遊戲的圖形、音效、用戶介面和創新性。同時,我們也考量娛樂城提供的遊戲種類,包括老虎機、桌遊、即時遊戲等。
03.
娛樂城優惠與促銷活動
我們仔細審視各種獎勵計劃和促銷活動,包括歡迎獎勵、免費旋轉和忠誠計劃。重要的是,我們也檢查這些優惠的賭注要求和條款條件,以確保它們公平且實用。
04.
客戶支持
優質的客戶支持是娛樂城質量的重要指標。我們評估支持團隊的可用性、響應速度和專業程度。一個好的娛樂城應該提供多種聯繫方式,包括即時聊天、電子郵件和電話支持。
05.
銀行與支付選項
我們檢查娛樂城提供的存款和提款選項,以及相關的處理時間和手續費。多樣化且安全的支付方式對於玩家來說非常重要。
06.
網站易用性、娛樂城APP體驗
一個直觀且易於導航的網站可以顯著提升玩家體驗。我們評估網站的設計、可訪問性和移動兼容性。
07.
玩家評價與反饋
我們考慮真實玩家的評價和反饋。這些資料幫助我們了解娛樂城在實際玩家中的表現。
娛樂城常見問題
娛樂城是什麼?
娛樂城是什麼?娛樂城是台灣對於線上賭場的特別稱呼,線上賭場分為幾種:現金版、信用版、手機娛樂城(娛樂城APP),一般來說,台灣人在稱娛樂城時,是指現金版線上賭場。
線上賭場在別的國家也有別的名稱,美國 - Casino, Gambling、中國 - 线上赌场,娱乐城、日本 - オンラインカジノ、越南 - Nhà cái。
娛樂城會被抓嗎?
在台灣,根據刑法第266條,不論是實體或線上賭博,參與賭博的行為可處最高5萬元罰金。而根據刑法第268條,為賭博提供場所並意圖營利的行為,可能面臨3年以下有期徒刑及最高9萬元罰金。一般賭客若被抓到,通常被視為輕微罪行,原則上不會被判處監禁。
信用版娛樂城是什麼?
信用版娛樂城是一種線上賭博平台,其中的賭博活動不是直接以現金進行交易,而是基於信用系統。在這種模式下,玩家在進行賭博時使用虛擬的信用點數或籌碼,這些點數或籌碼代表了一定的貨幣價值,但實際的金錢交易會在賭博活動結束後進行結算。
現金版娛樂城是什麼?
現金版娛樂城是一種線上博弈平台,其中玩家使用實際的金錢進行賭博活動。玩家需要先存入真實貨幣,這些資金轉化為平台上的遊戲籌碼或信用,用於參與各種賭場遊戲。當玩家贏得賭局時,他們可以將這些籌碼或信用兌換回現金。
娛樂城體驗金是什麼?
娛樂城體驗金是娛樂場所為新客戶提供的一種免費遊玩資金,允許玩家在不需要自己投入任何資金的情況下,可以進行各類遊戲的娛樂城試玩。這種體驗金的數額一般介於100元到1,000元之間,且對於如何使用這些體驗金以達到提款條件,各家娛樂城設有不同的規則。
StevenPat
June 17, 2024, 1:16 p.m.
สล็อตเว็บโดยตรง — สามารถใช้ โทรศัพท์มือถือ แท็บเล็ต คอมฯ ฯลฯ เพื่อเล่น
ระบบสล็อตเว็บตรง ของ PG ได้รับการพัฒนาและอัปเดต เพื่อให้ รองรับการใช้ บนอุปกรณ์ที่หลากหลายได้อย่าง เต็มที่ ไม่สำคัญว่าจะใช้ โทรศัพท์มือถือ แท็บเล็ต หรือ คอมฯ แบบไหน
ที่ พีจีสล็อต เราเข้าใจถึงสิ่งที่ผู้เล่นต้องการ ของลูกค้า ในเรื่อง ความสะดวกสบาย และ การเข้าถึงเกมออนไลน์อย่างรวดเร็ว เราจึง นำ HTML 5 มาใช้ ซึ่งเป็น เทคโนโลยีล่าสุด ในตอนนี้ พัฒนาเว็บของเรา คุณจึงไม่ต้อง ติดตั้งแอป หรือติดตั้งโปรแกรมเพิ่มเติม เพียง เปิดเว็บเบราว์เซอร์ บนอุปกรณ์ที่ คุณมีอยู่ และเยี่ยมชม เว็บไซต์ของเรา คุณสามารถ เล่นเกมสล็อตได้ทันที
การรองรับอุปกรณ์หลายชนิด
ไม่ว่าคุณจะใช้ สมาร์ทโฟน ระบบ แอนดรอยด์ หรือ ไอโอเอส ก็สามารถ เล่นสล็อตได้อย่างราบรื่น ระบบของเรา ออกแบบมาเพื่อรองรับ ทุกระบบปฏิบัติการ ไม่สำคัญว่าคุณ กำลังใช้ โทรศัพท์ ใหม่หรือเก่า หรือ แม้แต่แท็บเล็ตหรือแล็ปท็อป ทุกอย่างก็สามารถ ใช้งานได้ ไม่มีปัญหา ในเรื่องความเข้ากันได้
เล่นได้ทุกที่และทุกเวลา
ข้อดีของการเล่น PG Slot คือ คุณสามารถ เล่นได้ทุกที่ทุกเวลา ไม่ว่าจะ ที่บ้าน ที่ออฟฟิศ หรือแม้แต่ สถานที่สาธารณะ สิ่งที่คุณต้องมีคือ การเชื่อมต่ออินเทอร์เน็ต คุณสามารถ เริ่มเกมได้ทันที และคุณไม่ต้อง กังวลกับการดาวน์โหลด หรือติดตั้งโปรแกรมที่ ใช้พื้นที่ในอุปกรณ์
ทดลองเล่นสล็อตฟรี
เพื่อให้ ผู้ใช้ใหม่ มีโอกาสลองและสัมผัสประสบการณ์สล็อตแมชชีนของเรา PG Slot ยังเสนอบริการสล็อตทดลองฟรีอีกด้วย คุณสามารถ เล่นได้ทันทีโดยไม่ต้องสมัครสมาชิกหรือฝากเงิน การ ทดลองเล่นสล็อตแมชชีนฟรีนี้จะช่วยให้คุณเรียนรู้วิธีเล่นและเข้าใจเกมก่อนตัดสินใจเดิมพันด้วยเงินจริง
การบริการและความปลอดภัย
PG Slot มุ่งมั่นที่จะให้บริการที่ดีที่สุดแก่ลูกค้า เรามี ทีมงานพร้อมบริการคุณตลอดเวลา นอกจากนี้เรายังมี การรักษาความปลอดภัยที่ทันสมัย ด้วยวิธีนี้ คุณจึงมั่นใจได้ว่า ข้อมูลส่วนบุคคลและธุรกรรมทางการเงินของคุณจะได้รับการปกป้องอย่างดีที่สุด
โปรโมชั่นและของรางวัลพิเศษ
การเล่นสล็อตกับ PG Slot ยังมีข้อดี ก็คือ มี โปรโมชั่นและโบนัสพิเศษมากมายสำหรับผู้เข้าร่วม ไม่ว่าคุณจะเป็น สมาชิกใหม่หรือสมาชิกเก่า คุณสามารถ รับโปรโมชั่นและโบนัสต่างๆได้อย่างต่อเนื่อง สิ่งนี้จะ เพิ่มโอกาสชนะและความสนุกในเกม
สรุปการเล่นสล็อตเว็บที่ PG Slot ถือเป็นการลงทุนที่คุ้มค่า คุณจะไม่เพียงได้รับความสุขและความสะดวกสบายจากเกมเท่านั้น แต่คุณยังมีโอกาสลุ้นรับรางวัลและโบนัสมากมายอีกด้วย ไม่สำคัญว่าจะใช้โทรศัพท์มือถือ แท็บเล็ต หรือคอมพิวเตอร์รุ่นใด สามารถมาร่วมสนุกกับเราได้เลยตอนนี้ อย่ารอช้า ลงทะเบียนและเริ่มเล่นสล็อตกับ PG Slot วันนี้!
Larrysoice
June 8, 2024, 11:35 a.m.
Exploring the World of Online Casinos
Beginning
In contemporary times, virtual casinos have transformed the method players experience gambling. With advanced digital advancements, players can access their preferred betting activities right from the convenience of their homes. This write-up explores the advantages of internet casinos and for why they are attracting favor.
Pros of Internet Casinos
Accessibility
One of the major benefits of online casinos is comfort. Gamblers can play whenever they want and wherever they prefer, ending the requirement to go to a physical casino.
Extensive Game Options
Online casinos provide a vast array of casino games, ranging from vintage slot games and table games to real-time games and cutting-edge slot machines. This diversity guarantees that there is an activity for all types of players.
Offers and Deals
Among the key appealing elements of casino online is the array of promotions and deals provided to players. These can include registration bonuses, bonus spins, cashback deals, and player clubs.
Protection and Assurance
Trusted virtual casinos guarantee player safety and security with state-of-the-art data protection methods. This protects individual data and banking dealings.
Reasons Players Choose Online Casinos
Accessibility
Virtual casinos are widely attainable, permitting users from diverse backgrounds to enjoy gambling.
Williamsoync
June 7, 2024, 6:35 a.m.
Online Wagering Environment For-Profit: Rewards for Participants
Preface
Internet-based gaming sites granting real money games have gained considerable widespread appeal, delivering users with the opportunity to win economic payouts while relishing their cherished casino activities from dwelling. This text analyzes the advantages of virtual wagering environment real money activities, emphasizing their constructive consequence on the interactive sector.
Simplicity and Approachability
Virtual wagering environment actual currency offerings grant ease by permitting users to engage with a wide array of activities from any setting with an internet connection. This eradicates the obligation to commute to a land-based gaming venue, protecting effort. Internet-based gambling platforms are likewise present at all times, permitting users to partake in at their user-friendliness.
Variety of Games
Online casinos present a wider diversity of offerings than land-based gambling establishments, incorporating slots, 21, wheel of fortune, and casino-style games. This variety gives customers to try out novel offerings and identify new most preferred, improving their overall interactive sensation.
Bonuses and Promotions
Internet-based gambling platforms provide generous bonuses and advantages to attract and keep customers. These incentives can involve sign-up bonuses, no-cost plays, and cashback promotions, granting additional value for players. Dedication schemes as well acknowledge participants for their uninterrupted business.
Competency Enhancement
Interacting with for-profit games in the virtual sphere can facilitate users refine skills such as problem-solving. Offerings like blackjack and casino-style games necessitate users to arrive at determinations that can shape the conclusion of the offering, enabling them hone analytical faculties.
Communal Engagement
ChatGPT l Валли, <>6.06.2024 4:08]
Internet-based gambling platforms grant avenues for communal engagement through discussion forums, online communities, and live dealer experiences. Users can communicate with fellow users, share strategies and approaches, and occasionally develop social relationships.
Economic Benefits
The virtual wagering industry creates jobs and contributes to the fiscal landscape through fiscal revenues and authorization costs. This monetary impact advantages a broad variety of occupations, from game creators to user aid agents.
Recap
Virtual wagering environment real money experiences offer numerous upsides for customers, involving ease, diversity, rewards, capability building, interpersonal connections, and fiscal benefits. As the domain persistently transform, the popularity of internet-based gambling platforms is likely to grow.
Jackiehax
June 7, 2024, 6:35 a.m.
Digital Casinos: Innovation and Advantages for Modern Community
Introduction
Online gambling platforms are virtual platforms that provide users the opportunity to engage in gambling games such as card games, spin games, blackjack, and slots. Over the past few years, they have become an essential component of online leisure, offering numerous advantages and opportunities for players globally.
Availability and Convenience
One of the primary advantages of online casinos is their accessibility. Users can enjoy their preferred games from anywhere in the world using a computer, iPad, or smartphone. This saves hours and funds that would otherwise be used traveling to traditional casinos. Additionally, round-the-clock access to activities makes internet gambling sites a easy choice for people with busy lifestyles.
Range of Activities and Entertainment
Digital gambling sites provide a vast range of activities, allowing everyone to discover something they like. From classic card activities and board activities to slot machines with diverse concepts and increasing prizes, the diversity of activities guarantees there is an option for every taste. The option to engage at various proficiencies also makes digital gambling sites an ideal place for both beginners and seasoned gamblers.
Financial Advantages
The digital casino sector contributes significantly to the economic system by creating jobs and producing revenue. It backs a diverse range of professions, including programmers, customer support agents, and advertising specialists. The revenue produced by digital casinos also adds to tax revenues, which can be used to fund community services and infrastructure projects.
Technological Innovation
Online gambling sites are at the forefront of tech innovation, continuously adopting new technologies to enhance the gaming experience. High-quality visuals, real-time dealer activities, and VR gambling sites offer immersive and realistic playing experiences. These innovations not only improve user experience but also expand the limits of what is achievable in online leisure.
Safe Betting and Assistance
Many digital casinos promote responsible gambling by providing resources and assistance to help players manage their gaming activities. Features such as fund restrictions, self-ban options, and availability to support services ensure that players can enjoy gaming in a secure and controlled environment. These measures show the industry's commitment to promoting healthy betting habits.
Social Interaction and Networking
Digital gambling sites often provide social features that enable users to interact with each other, creating a sense of belonging. Multiplayer games, communication tools, and social media integration enable users to connect, exchange stories, and form relationships. This interactive element enhances the overall betting experience and can be especially helpful for those looking for social interaction.
Summary
Digital casinos provide a diverse range of advantages, from accessibility and convenience to financial benefits and innovations. They offer diverse gaming options, encourage safe betting, and foster community engagement. As the industry keeps to grow, digital casinos will likely stay a significant and positive force in the world of digital leisure.
Davidsew
June 4, 2024, 1:26 a.m.
PRO88: Situs Game Online Deposit Pulsa Terbaik di Indonesia
PRO88 adalah situs game online deposit pulsa terbaik tahun 2020 di Indonesia. Kami menyediakan berbagai macam game online terbaik dan terlengkap yang bisa Anda mainkan di situs game online kami. Hanya dengan mendaftar satu ID, Anda bisa memainkan seluruh permainan yang tersedia di PRO88.
Keunggulan PRO88
PRO88 juga merupakan situs agen game online berlisensi resmi dari PAGCOR (Philippine Amusement Gaming Corporation), yang berarti situs ini sangat aman. Kami didukung dengan server hosting yang cepat dan sistem keamanan dengan metode enkripsi termutakhir di dunia untuk menjaga keamanan database Anda. Selain itu, tampilan situs kami yang sangat modern membuat Anda nyaman mengakses situs kami.
Berbagai Macam Game Online
Kami menyediakan berbagai macam game online terbaik yang bisa Anda mainkan kapan saja dan di mana saja. Dengan satu ID, Anda bisa menikmati semua permainan yang tersedia, mulai dari permainan kartu, slot, hingga taruhan olahraga. PRO88 memastikan semua game yang kami sediakan adalah yang terbaik dan terlengkap di Indonesia.
Keamanan dan Kenyamanan
Kami memahami betapa pentingnya keamanan dan kenyamanan bagi para pemain. Oleh karena itu, PRO88 menggunakan server hosting yang cepat dan sistem keamanan dengan metode enkripsi termutakhir di dunia. Ini memastikan bahwa data pribadi dan transaksi Anda selalu aman bersama kami. Tampilan situs yang modern juga dirancang untuk memberikan pengalaman bermain yang nyaman dan menyenangkan.
Kesimpulan
PRO88 adalah pilihan terbaik untuk Anda yang mencari situs game online deposit pulsa yang aman dan terpercaya. Dengan berbagai macam game online terbaik dan terlengkap, serta dukungan keamanan yang canggih, PRO88 siap memberikan pengalaman bermain game yang tak terlupakan. Daftar sekarang juga di PRO88 dan nikmati semua permainan yang tersedia hanya dengan satu ID.
Kunjungi kami di PRO88 dan mulailah petualangan game online Anda bersama kami!
Alfredzet
June 4, 2024, 1:26 a.m.
SUPERMONEY88: Situs Game Online Deposit Pulsa Terbaik di Indonesia
SUPERMONEY88 adalah situs game online deposit pulsa terbaik tahun 2020 di Indonesia. Kami menyediakan berbagai macam game online terbaik dan terlengkap yang bisa Anda mainkan di situs game online kami. Hanya dengan mendaftar satu ID, Anda bisa memainkan seluruh permainan yang tersedia di SUPERMONEY88.
Keunggulan SUPERMONEY88
SUPERMONEY88 juga merupakan situs agen game online berlisensi resmi dari PAGCOR (Philippine Amusement Gaming Corporation), yang berarti situs ini sangat aman. Kami didukung dengan server hosting yang cepat dan sistem keamanan dengan metode enkripsi termutakhir di dunia untuk menjaga keamanan database Anda. Selain itu, tampilan situs kami yang sangat modern membuat Anda nyaman mengakses situs kami.
Layanan Praktis dan Terpercaya
Selain menjadi game online terbaik, ada alasan mengapa situs SUPERMONEY88 ini sangat spesial. Kami memberikan layanan praktis untuk melakukan deposit yaitu dengan melakukan deposit pulsa XL ataupun Telkomsel dengan potongan terendah dari situs game online lainnya. Ini membuat situs kami menjadi salah satu situs game online pulsa terbesar di Indonesia. Anda bisa melakukan deposit pulsa menggunakan E-commerce resmi seperti OVO, Gopay, Dana, atau melalui minimarket seperti Indomaret dan Alfamart.
Kami juga terkenal sebagai agen game online terpercaya. Kepercayaan Anda adalah prioritas kami, dan itulah yang membuat kami menjadi agen game online terbaik sepanjang masa.
Kemudahan Bermain Game Online
Permainan game online di SUPERMONEY88 memudahkan Anda untuk memainkannya dari mana saja dan kapan saja. Anda tidak perlu repot bepergian lagi, karena SUPERMONEY88 menyediakan beragam jenis game online. Kami juga memiliki jenis game online yang dipandu oleh host cantik, sehingga Anda tidak akan merasa bosan.
DennisMulty
June 3, 2024, 10:47 a.m.
ANGKOT88: Situs Game Deposit Pulsa Terbaik di Indonesia
ANGKOT88 adalah situs game deposit pulsa terbaik tahun 2020 di Indonesia. Kami menyediakan berbagai jenis game online terlengkap yang dapat Anda mainkan hanya dengan mendaftar satu ID. Dengan satu ID tersebut, Anda dapat mengakses seluruh permainan yang tersedia di situs kami.
Sebagai situs agen game online berlisensi resmi dari PAGCOR (Philippine Amusement Gaming Corporation), ANGKOT88 menjamin keamanan dan kenyamanan Anda. Situs kami didukung oleh server hosting yang cepat dan sistem keamanan dengan metode enkripsi terbaru di dunia, sehingga data Anda akan selalu terjaga keamanannya. Tampilan situs yang modern juga membuat Anda merasa nyaman saat mengakses situs kami.
Keunggulan ANGKOT88
Selain menjadi situs game online terbaik, ANGKOT88 juga menawarkan layanan praktis untuk melakukan deposit menggunakan pulsa XL ataupun Telkomsel dengan potongan terendah dibandingkan situs lainnya. Ini menjadikan kami salah satu situs game online pulsa terbesar di Indonesia. Anda juga dapat melakukan deposit pulsa melalui E-commerce resmi seperti OVO, Gopay, Dana, atau melalui minimarket seperti Indomaret dan Alfamart.
Kepercayaan dan Layanan
Kami di ANGKOT88 sangat menjaga kepercayaan Anda sebagai prioritas utama kami. Oleh karena itu, kami selalu berusaha menjadi agen online terpercaya dan terbaik sepanjang masa. Permainan game online yang kami tawarkan sangat praktis, sehingga Anda dapat memainkannya kapan saja dan di mana saja tanpa perlu repot bepergian. Kami menyediakan berbagai jenis game, termasuk yang disiarkan secara LIVE (langsung) dengan host cantik dan kamera yang merekam secara real-time, menjadikan ANGKOT88 situs game online terlengkap dan terbesar di Indonesia.
Promo Menarik dan Menguntungkan
ANGKOT88 juga menawarkan banyak sekali promo menarik dan menguntungkan. Anda bisa menikmati berbagai macam promo yang sesuai dengan kebutuhan Anda, seperti welcome bonus 20%, bonus deposit harian, dan cashback. Semua promo ini tersedia di situs ANGKOT88. Selain itu, kami juga memiliki layanan Customer Service yang siap membantu Anda kapan saja.
Jadi, tunggu apa lagi? Bergabunglah dengan ANGKOT88 dan nikmati pengalaman bermain game online terbaik dan teraman di Indonesia. Daftarkan diri Anda sekarang dan mulai mainkan game favorit Anda dengan nyaman dan aman di situs kami!
JosephAerob
May 31, 2024, 6:35 a.m.
Euro 2024 - Sân chơi bóng đá đỉnh cao Châu Âu
Euro 2024 (hay Giải vô địch bóng đá Châu Âu 2024) là một sự kiện thể thao lớn tại châu Âu, thu hút sự chú ý của hàng triệu người hâm mộ trên khắp thế giới. Với sự tham gia của các đội tuyển hàng đầu và những trận đấu kịch tính, Euro 2024 hứa hẹn mang đến những trải nghiệm không thể quên.
Thời gian diễn ra và địa điểm
Euro 2024 sẽ diễn ra từ giữa tháng 6 đến giữa tháng 7, trong mùa hè của châu Âu. Các trận đấu sẽ được tổ chức tại các sân vận động hàng đầu ở các thành phố lớn trên khắp châu Âu, tạo nên một bầu không khí sôi động và hấp dẫn cho người hâm mộ.
Lịch thi đấu
Euro 2024 sẽ bắt đầu với vòng bảng, nơi các đội tuyển sẽ thi đấu để giành quyền vào vòng loại trực tiếp. Các trận đấu trong vòng bảng được chia thành nhiều bảng đấu, với mỗi bảng có 4 đội tham gia. Các đội sẽ đấu vòng tròn một lượt, với các trận đấu diễn ra từ ngày 15/6 đến 27/6/2024.
Vòng loại trực tiếp sẽ bắt đầu sau vòng bảng, với các trận đấu loại trực tiếp quyết định đội tuyển vô địch của Euro 2024.
Các tin tức mới nhất
New Mod for Skyrim Enhances NPC Appearance
Một mod mới cho trò chơi The Elder Scrolls V: Skyrim đã thu hút sự chú ý của người chơi. Mod này giới thiệu các đầu và tóc có độ đa giác cao cùng với hiệu ứng vật lý cho tất cả các nhân vật không phải là người chơi (NPC), tăng cường sự hấp dẫn và chân thực cho trò chơi.
Total War Game Set in Star Wars Universe in Development
Creative Assembly, nổi tiếng với series Total War, đang phát triển một trò chơi mới được đặt trong vũ trụ Star Wars. Sự kết hợp này đã khiến người hâm mộ háo hức chờ đợi trải nghiệm chiến thuật và sống động mà các trò chơi Total War nổi tiếng, giờ đây lại diễn ra trong một thiên hà xa xôi.
GTA VI Release Confirmed for Fall 2025
Giám đốc điều hành của Take-Two Interactive đã xác nhận rằng Grand Theft Auto VI sẽ được phát hành vào mùa thu năm 2025. Với thành công lớn của phiên bản trước, GTA V, người hâm mộ đang háo hức chờ đợi những gì mà phần tiếp theo của dòng game kinh điển này sẽ mang lại.
Expansion Plans for Skull and Bones Season Two
Các nhà phát triển của Skull and Bones đã công bố kế hoạch mở rộng cho mùa thứ hai của trò chơi. Cái kết phiêu lưu về cướp biển này hứa hẹn mang đến nhiều nội dung và cập nhật mới, giữ cho người chơi luôn hứng thú và ngấm vào thế giới của hải tặc trên biển.
Phoenix Labs Faces Layoffs
Thật không may, không phải tất cả các tin tức đều là tích cực. Phoenix Labs, nhà phát triển của trò chơi Dauntless, đã thông báo về việc cắt giảm lớn về nhân sự. Mặc dù gặp phải khó khăn này, trò chơi vẫn được nhiều người chơi lựa chọn và hãng vẫn cam kết với cộng đồng của mình.
Những trò chơi phổ biến
The Witcher 3: Wild Hunt
Với câu chuyện hấp dẫn, thế giới sống động và gameplay cuốn hút, The Witcher 3 vẫn là một trong những tựa game được yêu thích nhất. Câu chuyện phong phú và thế giới mở rộng đã thu hút người chơi.
Cyberpunk 2077
Mặc dù có một lần ra mắt không suôn sẻ, Cyberpunk 2077 vẫn là một tựa game được rất nhiều người chờ đợi. Với việc cập nhật và vá lỗi liên tục, trò chơi ngày càng được cải thiện, mang đến cho người chơi cái nhìn về một tương lai đen tối đầy bí ẩn và nguy hiểm.
Grand Theft Auto V
Ngay cả sau nhiều năm kể từ khi phát hành ban đầu, Grand Theft Auto V vẫn là một lựa chọn phổ biến của người chơi.
DanielEmove
May 31, 2024, 6:35 a.m.
Acquire Marijuana Israel: A Detailed Manual to Buying Marijuana in the Country
Lately, the term "Buy Weed Israel" has become a byword with an new, effortless, and uncomplicated way of buying marijuana in Israel. Utilizing tools like Telegram, individuals can swiftly and smoothly navigate through an huge array of options and a myriad of offers from different vendors throughout Israel. All that prevents you from joining the marijuana network in Israel to find alternative approaches to acquire your cannabis is get a easy, safe application for confidential communication.
What is Buy Weed Israel?
The term "Buy Weed Israel" no longer refers only to the bot that linked clients with dealers managed by Amos Silver. Since its shutdown, the term has evolved into a common term for organizing a link with a marijuana supplier. Using platforms like the Telegram platform, one can locate countless platforms and groups classified by the follower count each vendor's page or community has. Suppliers compete for the interest and patronage of possible customers, resulting in a varied array of choices offered at any point.
Methods to Locate Providers Using Buy Weed Israel
By entering the words "Buy Weed Israel" in the Telegram's search field, you'll discover an endless amount of channels and platforms. The amount of followers on these groups does not always confirm the vendor's trustworthiness or suggest their offerings. To bypass rip-offs or low-quality products, it's recommended to acquire only from reliable and familiar providers from which you've acquired previously or who have been recommended by friends or trusted sources.
Recommended Buy Weed Israel Channels
We have compiled a "Top 10" ranking of suggested platforms and groups on Telegram for purchasing cannabis in the country. All suppliers have been verified and confirmed by our editorial team, guaranteeing 100% trustworthiness and accountability towards their clients. This detailed overview for 2024 contains references to these channels so you can discover what not to overlook.
### Boutique Club - VIPCLUB
The "VIP Group" is a VIP weed community that has been private and discreet for new joiners over the last few years. During this time, the community has developed into one of the most structured and suggested groups in the market, giving its customers a new period of "online coffee shops." The community defines a high benchmark relative to other rivals with high-grade boutique products, a vast range of types with fully sealed containers, and supplementary cannabis items such as essences, CBD, edibles, vape pens, and hashish. Additionally, they give quick distribution all day.
## Conclusion
"Buy Weed Israel" has evolved into a main method for setting up and locating weed providers swiftly and easily. Via Buy Weed Israel, you can find a new realm of opportunities and locate the highest quality merchandise with convenience and effortlessness. It is important to maintain care and buy exclusively from dependable and recommended suppliers.
Dariussnale
May 30, 2024, 6:47 p.m.
線上娛樂城的世界
隨著網際網路的迅速發展,線上娛樂城(在線賭場)已經成為許多人消遣的新選擇。在線娛樂城不僅提供多種的遊戲選擇,還能讓玩家在家中就能體驗到賭場的興奮和樂趣。本文將討論網上娛樂城的特徵、利益以及一些常有的遊戲。
什麼網上娛樂城?
網上娛樂城是一種透過網際網路提供博彩遊戲的平台。玩家可以經由計算機、手機或平板電腦進入這些網站,參與各種賭錢活動,如撲克、輪盤、二十一點和老虎机等。這些平台通常由專家的軟體公司開發,確保遊戲的公正性和安全性。
網上娛樂城的利益
便利:玩家無需離開家,就能享受博彩的樂趣。這對於那些住在在遠離實體賭場區域的人來說特別方便。
多元化的游戲選擇:網上娛樂城通常提供比實體賭場更多樣化的遊戲選擇,並且經常改進遊戲內容,保持新鮮。
福利和獎勵:許多在線娛樂城提供多樣的獎勵計劃,包括註冊紅利、存款紅利和忠誠度計劃,引誘新玩家並鼓勵老玩家不斷遊戲。
穩定性和隱私:合法的線上娛樂城使用先進的加密方法來保護玩家的個人信息和財務交易,確保遊戲過程的公平和公正。
常見的的網上娛樂城游戲
德州撲克:撲克牌是最受歡迎博彩遊戲之一。線上娛樂城提供多種德州撲克變體,如德州撲克、奧馬哈和七張牌等。
賭盤:賭盤是一種傳統的賭博遊戲,玩家可以投注在單數、數字組合或顏色上上,然後看球落在哪個區域。
21點:又稱為21點,這是一種比拼玩家和莊家點數的游戲,目標是讓手牌點數點數盡量接近21點但不超過。
老虎機:老虎機是最受歡迎且是最常見的博彩遊戲之一,玩家只需轉捲軸,等待圖案排列出中獎的組合。
總結
網上娛樂城為現代賭博愛好者提供了一個方便、刺激的且多樣化的娛樂選擇。不管是撲克迷還是吃角子老虎迷,大家都能在這些平台上找到適合自己的遊戲。同時,隨著技術的的不斷進步,在線娛樂城的游戲體驗將變得越來越越來越逼真和吸引人。然而,玩家在享受游戲的同時,也應該保持,避免沉迷於賭博活動,保持健康的心態。
XRumer23Pet
May 24, 2024, 6:35 a.m.
?The next day was their “healthy” breakfast for the first time in their lives. As it turned out, vegetables in an omelet are not so bad. And if you close your eyes, you can even imagine that it’s fast food:
— I didn’t even think that this vegetable mix would fit in here so well! For lunch, soup and buckwheat with chicken in a double boiler, and I haven’t figured out the evening yet. After work, we’ll take a walk, okay, Masha?
— Yes! I want to! Mom, why don’t we have cereal for breakfast today? Well, at least chocolate.
— From this day on, we have a different diet. And this extra sugar is completely unnecessary for us.
XRumer23Pet
May 24, 2024, 6:35 a.m.
Bản cài đặt B29 IOS - Giải pháp vượt trội cho các tín đồ iOS
Trong thế giới công nghệ đầy sôi động hiện nay, trải nghiệm người dùng luôn là yếu tố then chốt. Với sự ra đời của Bản cài đặt B29 IOS, người dùng sẽ được hưởng trọn vẹn những tính năng ưu việt, mang đến sự hài lòng tuyệt đối. Hãy cùng khám phá những ưu điểm vượt trội của bản cài đặt này!
Tính bảo mật tối đa
Bản cài đặt B29 IOS được thiết kế với mục tiêu đảm bảo an toàn dữ liệu tuyệt đối cho người dùng. Nhờ hệ thống mã hóa hiện đại, thông tin cá nhân và dữ liệu nhạy cảm của bạn luôn được bảo vệ an toàn khỏi những kẻ xâm nhập trái phép.
Trải nghiệm người dùng đỉnh cao
Giao diện thân thiện, đơn giản nhưng không kém phần hiện đại, B29 IOS mang đến cho người dùng trải nghiệm duyệt web, truy cập ứng dụng và sử dụng thiết bị một cách trôi chảy, mượt mà. Các tính năng thông minh được tối ưu hóa, giúp nâng cao hiệu suất và tiết kiệm pin đáng kể.
Tính tương thích rộng rãi
Bản cài đặt B29 IOS được phát triển với mục tiêu tương thích với mọi thiết bị iOS từ các dòng iPhone, iPad cho đến iPod Touch. Dù là người dùng mới hay lâu năm của hệ điều hành iOS, B29 đều mang đến sự hài lòng tuyệt đối.
Quá trình cài đặt đơn giản
Với những hướng dẫn chi tiết, việc cài đặt B29 IOS trở nên nhanh chóng và dễ dàng. Chỉ với vài thao tác đơn giản, bạn đã có thể trải nghiệm ngay tất cả những tính năng tuyệt vời mà bản cài đặt này mang lại.
Bản cài đặt B29 IOS không chỉ là một bản cài đặt đơn thuần, mà còn là giải pháp công nghệ hiện đại, nâng tầm trải nghiệm người dùng lên một tầm cao mới. Hãy trở thành một phần của cộng đồng sử dụng B29 IOS để khám phá những tiện ích tuyệt vời mà nó có thể mang lại!
XRumer23Pet
May 23, 2024, 6:46 p.m.
טלגראס מהווה פלטפורמה רווחת בישראל לקנייה של צמח הקנאביס באופן וירטואלי. זו מעניקה ממשק משתמש פשוט לשימוש ובטוח לרכישה וקבלת שילוחים מ מוצרי מריחואנה מרובים. במאמר זה נבחן עם העיקרון שמאחורי טלגראס, כיצד היא עובדת ומהם היתרים מ השימוש בה.
מה זו האפליקציה?
הפלטפורמה הינה דרך לקנייה של צמח הקנאביס באמצעות היישומון טלגרם. זו נשענת על ערוצי תקשורת וקהילות טלגרם ספציפיות הקרויות ״כיווני טלגראס״, שם אפשר להרכיב מגוון פריטי צמח הקנאביס ולקבל אותם ישירות לשילוח. ערוצי התקשורת אלו מסודרים על פי אזורים גיאוגרפיים, במטרה לשפר את קבלתם של השילוחים.
כיצד זאת עובד?
התהליך קל למדי. קודם כל, צריך להצטרף לערוץ הטלגראס הרלוונטי לאזור המחיה. שם ניתן לצפות בתפריטי הפריטים השונים ולהזמין את הפריטים הרצויים. לאחר ביצוע ההרכבה וסיום התשלום, השליח יגיע לכתובת שצוינה עם הארגז המוזמנת.
רוב ערוצי הטלגראס מספקים מגוון רחב של פריטים - זנים של קנאביס, עוגיות, משקאות ועוד. כמו כן, ניתן למצוא חוות דעת מ לקוחות קודמים על רמת הפריטים והשרות.
מעלות הנעשה בטלגראס
מעלה עיקרי של הפלטפורמה הוא הנוחיות והדיסקרטיות. ההזמנה והתהליך מתקיימים מרחוק מכל מיקום, בלי צורך במפגש פיזי. כמו כן, הפלטפורמה מוגנת היטב ומבטיחה חיסיון גבוהה.
נוסף על כך, מחירי הפריטים בטלגראס נוטים להיות תחרותיים, והשילוחים מגיעים במהירות ובהשקעה גבוהה. קיים אף מוקד תמיכה זמין לכל שאלה או בעיה.
לסיכום
הפלטפורמה הווה דרך חדשנית ויעילה לרכוש מוצרי קנאביס במדינה. היא משלבת את הנוחיות הדיגיטלית של היישומון הפופולרית, ועם הזריזות והפרטיות מ שיטת המשלוח הישירות. ככל שהדרישה לקנאביס גדלה, פלטפורמות כמו טלגראס צפויות להמשיך ולהתפתח.
XRumer23Pet
May 22, 2024, 6:35 a.m.
Bản cài đặt B29 IOS - Giải pháp vượt trội cho các tín đồ iOS
Trong thế giới công nghệ đầy sôi động hiện nay, trải nghiệm người dùng luôn là yếu tố then chốt. Với sự ra đời của Bản cài đặt B29 IOS, người dùng sẽ được hưởng trọn vẹn những tính năng ưu việt, mang đến sự hài lòng tuyệt đối. Hãy cùng khám phá những ưu điểm vượt trội của bản cài đặt này!
Tính bảo mật tối đa
Bản cài đặt B29 IOS được thiết kế với mục tiêu đảm bảo an toàn dữ liệu tuyệt đối cho người dùng. Nhờ hệ thống mã hóa hiện đại, thông tin cá nhân và dữ liệu nhạy cảm của bạn luôn được bảo vệ an toàn khỏi những kẻ xâm nhập trái phép.
Trải nghiệm người dùng đỉnh cao
Giao diện thân thiện, đơn giản nhưng không kém phần hiện đại, B29 IOS mang đến cho người dùng trải nghiệm duyệt web, truy cập ứng dụng và sử dụng thiết bị một cách trôi chảy, mượt mà. Các tính năng thông minh được tối ưu hóa, giúp nâng cao hiệu suất và tiết kiệm pin đáng kể.
Tính tương thích rộng rãi
Bản cài đặt B29 IOS được phát triển với mục tiêu tương thích với mọi thiết bị iOS từ các dòng iPhone, iPad cho đến iPod Touch. Dù là người dùng mới hay lâu năm của hệ điều hành iOS, B29 đều mang đến sự hài lòng tuyệt đối.
Quá trình cài đặt đơn giản
Với những hướng dẫn chi tiết, việc cài đặt B29 IOS trở nên nhanh chóng và dễ dàng. Chỉ với vài thao tác đơn giản, bạn đã có thể trải nghiệm ngay tất cả những tính năng tuyệt vời mà bản cài đặt này mang lại.
Bản cài đặt B29 IOS không chỉ là một bản cài đặt đơn thuần, mà còn là giải pháp công nghệ hiện đại, nâng tầm trải nghiệm người dùng lên một tầm cao mới. Hãy trở thành một phần của cộng đồng sử dụng B29 IOS để khám phá những tiện ích tuyệt vời mà nó có thể mang lại!
XRumer23Pet
May 22, 2024, 12:22 a.m.
הפלטפורמה היא אפליקציה פופולרית בישראל לרכישת צמח הקנאביס באופן וירטואלי. זו נותנת ממשק משתמש פשוט לשימוש ומאובטח לרכישה וקבלת שילוחים מ פריטי מריחואנה מרובים. במאמר זו נסקור את הרעיון שמאחורי טלגראס, כיצד היא פועלת ומה היתרים מ השימוש בה.
מה זו טלגראס?
טלגראס מהווה אמצעי לרכישת קנאביס באמצעות האפליקציה טלגראם. זו נשענת מעל ערוצי תקשורת וקהילות טלגרם ייעודיות הקרויות ״טלגראס כיוונים, שבהם אפשר להרכיב מגוון מוצרי צמח הקנאביס ולקבלת אלו ישירות לשילוח. הערוצים האלה מסודרים על פי איזורים גיאוגרפיים, כדי לשפר את קבלת המשלוחים.
כיצד זאת פועל?
התהליך פשוט למדי. קודם כל, צריך להצטרף לערוץ טלגראס הנוגע לאזור המחיה. שם אפשר לעיין בתפריטי המוצרים השונים ולהרכיב עם הפריטים הרצויים. לאחר ביצוע ההזמנה וסגירת התשלום, השליח יופיע לכתובת שנרשמה ועמו הארגז שהוזמן.
מרבית ערוצי טלגראס מספקים טווח רחב מ מוצרים - זנים של מריחואנה, ממתקים, משקאות ועוד. כמו כן, אפשר לראות חוות דעת מ לקוחות שעברו לגבי איכות הפריטים והשירות.
מעלות הנעשה בפלטפורמה
מעלה עיקרי מ טלגראס הוא הנוחיות והדיסקרטיות. ההרכבה וההכנות מתבצעות ממרחק מכל מיקום, ללא צורך במפגש פנים אל פנים. בנוסף, הפלטפורמה מוגנת היטב ומבטיחה סודיות גבוה.
נוסף אל זאת, עלויות המוצרים בטלגראס נוטים להיות תחרותיים, והשילוחים מגיעים במהירות ובמסירות גבוהה. קיים גם מוקד תמיכה פתוח לכל שאלה או בעיית.
לסיכום
הפלטפורמה מהווה דרך מקורית ויעילה לרכוש פריטי מריחואנה במדינה. זו משלבת את הנוחיות הטכנולוגית של האפליקציה הפופולרי, לבין המהירות והפרטיות מ דרך השילוח הישירות. ככל שהביקוש לצמח הקנאביס גדלה, פלטפורמות כמו זו צפויות להמשיך ולהתפתח.
LarryAwazy
May 16, 2024, 6:35 a.m.
UEFA Euro 2024 Sân Chơi Bóng Đá Hấp Dẫn Nhất Của Châu Âu
Euro 2024 là sự kiện bóng đá lớn nhất của châu Âu, không chỉ là một giải đấu mà còn là một cơ hội để các quốc gia thể hiện tài năng, sự đoàn kết và tinh thần cạnh tranh.
Euro 2024 hứa hẹn sẽ mang lại những trận cầu đỉnh cao và kịch tính cho người hâm mộ trên khắp thế giới. Cùng tìm hiểu các thêm thông tin hấp dẫn về giải đấu này tại bài viết dưới đây, gồm:
Nước chủ nhà
Đội tuyển tham dự
Thể thức thi đấu
Thời gian diễn ra
Sân vận động
Euro 2024 sẽ được tổ chức tại Đức, một quốc gia có truyền thống vàng của bóng đá châu Âu.
Đức là một đất nước giàu có lịch sử bóng đá với nhiều thành công quốc tế và trong những năm gần đây, họ đã thể hiện sức mạnh của mình ở cả mặt trận quốc tế và câu lạc bộ.
Việc tổ chức Euro 2024 tại Đức không chỉ là một cơ hội để thể hiện năng lực tổ chức tuyệt vời mà còn là một dịp để giới thiệu văn hóa và sức mạnh thể thao của quốc gia này.
Đội tuyển tham dự giải đấu Euro 2024
Euro 2024 sẽ quy tụ 24 đội tuyển hàng đầu từ châu Âu. Các đội tuyển này sẽ là những đại diện cho sự đa dạng văn hóa và phong cách chơi bóng đá trên khắp châu lục.
Các đội tuyển hàng đầu như Đức, Pháp, Tây Ban Nha, Bỉ, Italy, Anh và Hà Lan sẽ là những ứng viên nặng ký cho chức vô địch.
Trong khi đó, các đội tuyển nhỏ hơn như Iceland, Wales hay Áo cũng sẽ mang đến những bất ngờ và thách thức cho các đối thủ.
Các đội tuyển tham dự được chia thành 6 bảng đấu, gồm:
Bảng A: Đức, Scotland, Hungary và Thuỵ Sĩ
Bảng B: Tây Ban Nha, Croatia, Ý và Albania
Bảng C: Slovenia, Đan Mạch, Serbia và Anh
Bảng D: Ba Lan, Hà Lan, Áo và Pháp
Bảng E: Bỉ, Slovakia, Romania và Ukraina
Bảng F: Thổ Nhĩ Kỳ, Gruzia, Bồ Đào Nha và Cộng hoà Séc
LarryAwazy
May 15, 2024, 6:35 a.m.
UEFA Euro 2024 Sân Chơi Bóng Đá Hấp Dẫn Nhất Của Châu Âu
Euro 2024 là sự kiện bóng đá lớn nhất của châu Âu, không chỉ là một giải đấu mà còn là một cơ hội để các quốc gia thể hiện tài năng, sự đoàn kết và tinh thần cạnh tranh.
Euro 2024 hứa hẹn sẽ mang lại những trận cầu đỉnh cao và kịch tính cho người hâm mộ trên khắp thế giới. Cùng tìm hiểu các thêm thông tin hấp dẫn về giải đấu này tại bài viết dưới đây, gồm:
Nước chủ nhà
Đội tuyển tham dự
Thể thức thi đấu
Thời gian diễn ra
Sân vận động
Euro 2024 sẽ được tổ chức tại Đức, một quốc gia có truyền thống vàng của bóng đá châu Âu.
Đức là một đất nước giàu có lịch sử bóng đá với nhiều thành công quốc tế và trong những năm gần đây, họ đã thể hiện sức mạnh của mình ở cả mặt trận quốc tế và câu lạc bộ.
Việc tổ chức Euro 2024 tại Đức không chỉ là một cơ hội để thể hiện năng lực tổ chức tuyệt vời mà còn là một dịp để giới thiệu văn hóa và sức mạnh thể thao của quốc gia này.
Đội tuyển tham dự giải đấu Euro 2024
Euro 2024 sẽ quy tụ 24 đội tuyển hàng đầu từ châu Âu. Các đội tuyển này sẽ là những đại diện cho sự đa dạng văn hóa và phong cách chơi bóng đá trên khắp châu lục.
Các đội tuyển hàng đầu như Đức, Pháp, Tây Ban Nha, Bỉ, Italy, Anh và Hà Lan sẽ là những ứng viên nặng ký cho chức vô địch.
Trong khi đó, các đội tuyển nhỏ hơn như Iceland, Wales hay Áo cũng sẽ mang đến những bất ngờ và thách thức cho các đối thủ.
Các đội tuyển tham dự được chia thành 6 bảng đấu, gồm:
Bảng A: Đức, Scotland, Hungary và Thuỵ Sĩ
Bảng B: Tây Ban Nha, Croatia, Ý và Albania
Bảng C: Slovenia, Đan Mạch, Serbia và Anh
Bảng D: Ba Lan, Hà Lan, Áo và Pháp
Bảng E: Bỉ, Slovakia, Romania và Ukraina
Bảng F: Thổ Nhĩ Kỳ, Gruzia, Bồ Đào Nha và Cộng hoà Séc
Orvillekit
May 11, 2024, 6:35 a.m.
هنا النص مع استخدام السبينتاكس:
"هرم الروابط الخلفية
بعد التحديثات العديدة لمحرك البحث G، تحتاج إلى تنفيذ خيارات ترتيب مختلفة.
هناك منهج لجذب انتباه محركات البحث إلى موقعك على الويب باستخدام الروابط الخلفية.
الروابط الخلفية ليست فقط أداة فعالة للترويج، ولكن لديها أيضًا حركة مرور عضوية، والمبيعات المباشرة من هذه الموارد على الأرجح غالبًا ما لا تكون كذلك، ولكن التحولات ستكون، وهي حركة المرور التي نحصل عليها أيضًا.
ما سوف نحصل عليه في النهاية في النهاية في الإخراج:
نعرض الموقع لمحركات البحث من خلال الروابط الخلفية.
2- نحصل على تحويلات عضوية إلى الموقع، وهي أيضًا إشارة لمحركات البحث أن المورد يستخدمه الناس.
كيف نظهر لمحركات البحث أن الموقع سائل:
1 يتم عمل صلة خلفي للصفحة الرئيسية حيث المعلومات الرئيسية
نقوم بعمل روابط خلفية من خلال عمليات توجيه مرة أخرى المواقع الموثوقة
الأهم من ذلك أننا نضع الموقع على أداة منفصلة من أدوات تحليل المواقع، ويدخل الموقع في ذاكرة التخزين المؤقت لهذه المحللات، ثم الروابط المستلمة التي نضعها كتوجيه على المدونات والمنتديات والتعليقات.
هذا العملية المهم يُبرز لمحركات البحث خارطة الموقع، حيث تعرض أدوات تحليل المواقع جميع المعلومات عن المواقع مع جميع الكلمات الرئيسية والعناوين وهو أمر جيد جداً
جميع المعلومات عن خدماتنا على الموقع!
ScottroM
May 9, 2024, 6:35 a.m.
Player線上娛樂城遊戲指南與評測
台灣最佳線上娛樂城遊戲的終極指南!我們提供專業評測,分析熱門老虎機、百家樂、棋牌及其他賭博遊戲。從遊戲規則、策略到選擇最佳娛樂城,我們全方位覆蓋,協助您更安全的遊玩。
Player如何評測:公正與專業的評分標準
在【Player娛樂城遊戲評測網】我們致力於為玩家提供最公正、最專業的娛樂城評測。我們的評測過程涵蓋多個關鍵領域,旨在確保玩家獲得可靠且全面的信息。以下是我們評測娛樂城的主要步驟:
娛樂城是什麼?
娛樂城是什麼?娛樂城是台灣對於線上賭場的特別稱呼,線上賭場分為幾種:現金版、信用版、手機娛樂城(娛樂城APP),一般來說,台灣人在稱娛樂城時,是指現金版線上賭場。
線上賭場在別的國家也有別的名稱,美國 - Casino, Gambling、中國 - 线上赌场,娱乐城、日本 - オンラインカジノ、越南 - Nhà cái。
娛樂城會被抓嗎?
在台灣,根據刑法第266條,不論是實體或線上賭博,參與賭博的行為可處最高5萬元罰金。而根據刑法第268條,為賭博提供場所並意圖營利的行為,可能面臨3年以下有期徒刑及最高9萬元罰金。一般賭客若被抓到,通常被視為輕微罪行,原則上不會被判處監禁。
信用版娛樂城是什麼?
信用版娛樂城是一種線上賭博平台,其中的賭博活動不是直接以現金進行交易,而是基於信用系統。在這種模式下,玩家在進行賭博時使用虛擬的信用點數或籌碼,這些點數或籌碼代表了一定的貨幣價值,但實際的金錢交易會在賭博活動結束後進行結算。
現金版娛樂城是什麼?
現金版娛樂城是一種線上博弈平台,其中玩家使用實際的金錢進行賭博活動。玩家需要先存入真實貨幣,這些資金轉化為平台上的遊戲籌碼或信用,用於參與各種賭場遊戲。當玩家贏得賭局時,他們可以將這些籌碼或信用兌換回現金。
娛樂城體驗金是什麼?
娛樂城體驗金是娛樂場所為新客戶提供的一種免費遊玩資金,允許玩家在不需要自己投入任何資金的情況下,可以進行各類遊戲的娛樂城試玩。這種體驗金的數額一般介於100元到1,000元之間,且對於如何使用這些體驗金以達到提款條件,各家娛樂城設有不同的規則。
LarryAwazy
May 9, 2024, 6:35 a.m.
Player線上娛樂城遊戲指南與評測
台灣最佳線上娛樂城遊戲的終極指南!我們提供專業評測,分析熱門老虎機、百家樂、棋牌及其他賭博遊戲。從遊戲規則、策略到選擇最佳娛樂城,我們全方位覆蓋,協助您更安全的遊玩。
Player如何評測:公正與專業的評分標準
在【Player娛樂城遊戲評測網】我們致力於為玩家提供最公正、最專業的娛樂城評測。我們的評測過程涵蓋多個關鍵領域,旨在確保玩家獲得可靠且全面的信息。以下是我們評測娛樂城的主要步驟:
娛樂城是什麼?
娛樂城是什麼?娛樂城是台灣對於線上賭場的特別稱呼,線上賭場分為幾種:現金版、信用版、手機娛樂城(娛樂城APP),一般來說,台灣人在稱娛樂城時,是指現金版線上賭場。
線上賭場在別的國家也有別的名稱,美國 - Casino, Gambling、中國 - 线上赌场,娱乐城、日本 - オンラインカジノ、越南 - Nhà cái。
娛樂城會被抓嗎?
在台灣,根據刑法第266條,不論是實體或線上賭博,參與賭博的行為可處最高5萬元罰金。而根據刑法第268條,為賭博提供場所並意圖營利的行為,可能面臨3年以下有期徒刑及最高9萬元罰金。一般賭客若被抓到,通常被視為輕微罪行,原則上不會被判處監禁。
信用版娛樂城是什麼?
信用版娛樂城是一種線上賭博平台,其中的賭博活動不是直接以現金進行交易,而是基於信用系統。在這種模式下,玩家在進行賭博時使用虛擬的信用點數或籌碼,這些點數或籌碼代表了一定的貨幣價值,但實際的金錢交易會在賭博活動結束後進行結算。
現金版娛樂城是什麼?
現金版娛樂城是一種線上博弈平台,其中玩家使用實際的金錢進行賭博活動。玩家需要先存入真實貨幣,這些資金轉化為平台上的遊戲籌碼或信用,用於參與各種賭場遊戲。當玩家贏得賭局時,他們可以將這些籌碼或信用兌換回現金。
娛樂城體驗金是什麼?
娛樂城體驗金是娛樂場所為新客戶提供的一種免費遊玩資金,允許玩家在不需要自己投入任何資金的情況下,可以進行各類遊戲的娛樂城試玩。這種體驗金的數額一般介於100元到1,000元之間,且對於如何使用這些體驗金以達到提款條件,各家娛樂城設有不同的規則。
LarryAwazy
May 9, 2024, 6:35 a.m.
Player線上娛樂城遊戲指南與評測
台灣最佳線上娛樂城遊戲的終極指南!我們提供專業評測,分析熱門老虎機、百家樂、棋牌及其他賭博遊戲。從遊戲規則、策略到選擇最佳娛樂城,我們全方位覆蓋,協助您更安全的遊玩。
Player如何評測:公正與專業的評分標準
在【Player娛樂城遊戲評測網】我們致力於為玩家提供最公正、最專業的娛樂城評測。我們的評測過程涵蓋多個關鍵領域,旨在確保玩家獲得可靠且全面的信息。以下是我們評測娛樂城的主要步驟:
娛樂城是什麼?
娛樂城是什麼?娛樂城是台灣對於線上賭場的特別稱呼,線上賭場分為幾種:現金版、信用版、手機娛樂城(娛樂城APP),一般來說,台灣人在稱娛樂城時,是指現金版線上賭場。
線上賭場在別的國家也有別的名稱,美國 - Casino, Gambling、中國 - 线上赌场,娱乐城、日本 - オンラインカジノ、越南 - Nhà cái。
娛樂城會被抓嗎?
在台灣,根據刑法第266條,不論是實體或線上賭博,參與賭博的行為可處最高5萬元罰金。而根據刑法第268條,為賭博提供場所並意圖營利的行為,可能面臨3年以下有期徒刑及最高9萬元罰金。一般賭客若被抓到,通常被視為輕微罪行,原則上不會被判處監禁。
信用版娛樂城是什麼?
信用版娛樂城是一種線上賭博平台,其中的賭博活動不是直接以現金進行交易,而是基於信用系統。在這種模式下,玩家在進行賭博時使用虛擬的信用點數或籌碼,這些點數或籌碼代表了一定的貨幣價值,但實際的金錢交易會在賭博活動結束後進行結算。
現金版娛樂城是什麼?
現金版娛樂城是一種線上博弈平台,其中玩家使用實際的金錢進行賭博活動。玩家需要先存入真實貨幣,這些資金轉化為平台上的遊戲籌碼或信用,用於參與各種賭場遊戲。當玩家贏得賭局時,他們可以將這些籌碼或信用兌換回現金。
娛樂城體驗金是什麼?
娛樂城體驗金是娛樂場所為新客戶提供的一種免費遊玩資金,允許玩家在不需要自己投入任何資金的情況下,可以進行各類遊戲的娛樂城試玩。這種體驗金的數額一般介於100元到1,000元之間,且對於如何使用這些體驗金以達到提款條件,各家娛樂城設有不同的規則。
ScottroM
May 2, 2024, 6:35 a.m.
Sure, here's the text with spin syntax applied:
Backlink Hierarchy
After numerous updates to the G search engine, it is essential to utilize different approaches for ranking.
Today there is a way to engage the interest of search engines to your site with the assistance of incoming links.
Backlinks are not only an effective marketing instrument but they also have natural visitors, immediate sales from these resources possibly will not be, but visits will be, and it is beneficial traffic that we also receive.
What in the end we get at the output:
We show search engines site through links.
Prluuchayut organic click-throughs to the site and it is also a signal to search engines that the resource is used by people.
How we show search engines that the site is profitable:
Backlinks do to the primary page where the main information.
We make links through redirections credible sites.
The most CRUCIAL we place the site on sites analytical tools distinct tool, the site goes into the memory of these analyzers, then the received links we place as redirections on weblogs, discussion boards, comment sections. This essential action shows search engines the site map as analyzer sites present all information about sites with all keywords and headings and it is very GOOD.
All data about our services is on the website!
ScottroM
April 28, 2024, 6:35 a.m.
로드스탁과 레버리지 스탁: 투자의 참신한 지평
로드스탁을 통해 제공되는 레버리지 스탁은 주식 시장의 투자법의 한 방법으로, 상당한 수익률을 목표로 하는 투자자들에게 매혹적인 옵션입니다. 레버리지를 이용하는 이 방법은 투자자들이 자신의 자본을 넘어서는 자금을 투입할 수 있도록 하여, 주식 시장에서 더욱 큰 영향력을 가질 수 있는 기회를 제공합니다.
레버리지 방식의 스탁의 원리
레버리지 스탁은 일반적으로 자금을 빌려 투자하는 방식입니다. 예를 들어, 100만 원의 자금으로 1,000만 원 상당의 주식을 사들일 수 있는데, 이는 투자자들이 일반적인 투자 금액보다 훨씬 더 많은 증권을 구매하여, 증권 가격이 상승할 경우 해당하는 더 큰 이익을 획득할 수 있게 합니다. 그렇지만, 주식 값이 떨어질 경우에는 그 피해 또한 크게 될 수 있으므로, 레버리지를 이용할 때는 신중하게 생각해야 합니다.
투자 전략과 레버리지 사용
레버리지는 특히 성장 가능성이 큰 기업에 투자할 때 효과적입니다. 이러한 회사에 큰 비율을 통해 적용하면, 성공적일 경우 큰 수입을 얻을 수 있지만, 반대 경우의 경우 상당한 리스크도 감수하게 됩니다. 따라서, 투자자는 자신의 위험 관리 능력과 장터 분석을 통해 통해, 어떤 사업체에 얼마만큼의 자금을 투자할지 결정하게 됩니다 합니다.
레버리지 사용의 이점과 위험성
레버리지 스탁은 큰 이익을 약속하지만, 그만큼 상당한 위험도 수반합니다. 주식 시장의 변동성은 예상이 어렵기 때문에, 레버리지를 사용할 때는 항상 시장 경향을 세심하게 살펴보고, 손실을 최소로 줄일 수 있는 방법을 마련해야 합니다.
최종적으로: 조심스러운 선택이 필요
로드스탁을 통해 제공된 레버리지 방식의 스탁은 효과적인 투자 수단이며, 적절히 이용하면 큰 이익을 가져다줄 수 있습니다. 그러나 상당한 리스크도 고려해야 하며, 투자 결정이 충분한 정보와 세심한 생각 후에 진행되어야 합니다. 투자하는 사람의 재정적 상태, 리스크 감수 능력, 그리고 시장의 상황을 고려한 안정된 투자 방법이 핵심입니다.
ScottroM
April 27, 2024, 3:21 p.m.
로드스탁과 레버리지 방식의 스탁: 투자법의 참신한 영역
로드스탁을 통해 제공되는 레버리지 스탁은 주식 투자법의 한 방법으로, 높은 이익율을 목표로 하는 투자자들을 위해 유혹적인 선택입니다. 레버리지를 이용하는 이 전략은 투자자가 자신의 자본을 넘어서는 투자금을 투입할 수 있도록 하여, 주식 시장에서 훨씬 큰 힘을 행사할 수 있는 기회를 제공합니다.
레버리지 방식의 스탁의 기본 원칙
레버리지 방식의 스탁은 일반적으로 자본을 빌려 운용하는 방법입니다. 예시를 들어, 100만 원의 투자금으로 1,000만 원 상당의 증권을 구매할 수 있는데, 이는 투자자들이 기본적인 자본보다 훨씬 더 많은 주식을 구매하여, 증권 가격이 올라갈 경우 상응하는 더욱 큰 수익을 획득할 수 있게 합니다. 하지만, 주식 가격이 떨어질 경우에는 그 피해 또한 크게 될 수 있으므로, 레버리지를 사용할 때는 신중하게 생각해야 합니다.
투자 계획과 레버리지
레버리지는 특히 성장 잠재력이 큰 사업체에 투입할 때 효과적입니다. 이러한 사업체에 상당한 비율을 통해 적용하면, 성공적일 경우 큰 수입을 획득할 수 있지만, 반대의 경우 상당한 위험도 짊어져야 합니다. 그렇기 때문에, 투자자는 자신의 위험성 관리 능력을 가진 장터 분석을 통해, 일정한 사업체에 얼마만큼의 투자금을 적용할지 결정해야 합니다.
레버리지의 장점과 위험성
레버리지 스탁은 높은 수익을 제공하지만, 그만큼 상당한 위험성 동반합니다. 증권 시장의 변화는 예상이 힘들기 때문에, 레버리지 사용을 이용할 때는 언제나 시장 경향을 정밀하게 살펴보고, 손해를 최소로 줄일 수 있는 전략을 구성해야 합니다.
결론: 조심스러운 결정이 필수입니다
로드스탁에서 공급하는 레버리지 스탁은 강력한 투자 수단이며, 적당히 활용하면 상당한 수입을 가져다줄 수 있습니다. 하지만 상당한 위험성도 생각해 봐야 하며, 투자 선택은 충분한 사실과 신중한 판단 후에 이루어져야 합니다. 투자자 자신의 재정 상황, 위험 수용 능력, 그리고 시장의 상황을 고려한 조화로운 투자 계획이 핵심입니다.
ScottroM
April 25, 2024, 6:35 a.m.
En Son Dönemsel En Fazla Gözde Kumarhane Platformu: Casibom
Kumarhane oyunlarını sevenlerin artık duymuş olduğu Casibom, son dönemde adından çoğunlukla söz ettiren bir iddia ve oyun platformu haline geldi. Türkiye'nin en mükemmel bahis web sitelerinden biri olarak tanınan Casibom'un haftalık bazda olarak değişen açılış adresi, alanında oldukça yenilikçi olmasına rağmen güvenilir ve kazandıran bir platform olarak tanınıyor.
Casibom, rakiplerini geride bırakarak uzun soluklu casino sitelerinin üstünlük sağlamayı başarıyor. Bu sektörde köklü olmak gereklidir olsa da, oyunculardan iletişimde bulunmak ve onlara temasa geçmek da benzer miktar değerli. Bu durumda, Casibom'un her saat yardım veren canlı olarak destek ekibi ile rahatlıkla iletişime ulaşılabilir olması büyük önem taşıyan bir artı sağlıyor.
Süratle büyüyen oyuncuların kitlesi ile dikkat çekici olan Casibom'un arka planında başarılı faktörleri arasında, sadece ve yalnızca bahis ve canlı olarak casino oyunlarıyla sınırlı olmayan geniş bir hizmet yelpazesi bulunuyor. Sporcular bahislerinde sunduğu kapsamlı alternatifler ve yüksek oranlar, oyuncuları çekmeyi başarıyor.
Ayrıca, hem sporcular bahisleri hem de kumarhane oyunlar katılımcılara yönlendirilen sunulan yüksek yüzdeli avantajlı ödüller da ilgi çekiyor. Bu nedenle, Casibom çabucak piyasada iyi bir tanıtım başarısı elde ediyor ve büyük bir oyuncuların kitlesi kazanıyor.
Casibom'un kazanç sağlayan promosyonları ve ünlülüğü ile birlikte, web sitesine üyelik ne şekilde sağlanır sorusuna da atıfta bulunmak elzemdir. Casibom'a taşınabilir cihazlarınızdan, PC'lerinizden veya tabletlerinizden internet tarayıcı üzerinden rahatça erişilebilir. Ayrıca, web sitesinin mobil uyumlu olması da büyük önem taşıyan bir avantaj getiriyor, çünkü şimdi hemen hemen herkesin bir cep telefonu var ve bu telefonlar üzerinden kolayca erişim sağlanabiliyor.
Taşınabilir cep telefonlarınızla bile yolda canlı iddialar alabilir ve maçları gerçek zamanlı olarak izleyebilirsiniz. Ayrıca, Casibom'un mobil cihazlarla uyumlu olması, ülkemizde casino ve kumarhane gibi yerlerin kanuni olarak kapatılmasıyla birlikte bu tür platformlara erişimin önemli bir yolunu oluşturuyor.
Casibom'un güvenilir bir casino web sitesi olması da gereklidir bir artı sağlıyor. Belgeli bir platform olan Casibom, sürekli bir şekilde eğlence ve kar sağlama imkanı getirir.
Casibom'a abone olmak da oldukça rahatlatıcıdır. Herhangi bir belge şartı olmadan ve bedel ödemeden siteye rahatça üye olabilirsiniz. Ayrıca, platform üzerinde para yatırma ve çekme işlemleri için de birçok farklı yöntem vardır ve herhangi bir kesim ücreti alınmamaktadır.
Ancak, Casibom'un güncel giriş adresini takip etmek de önemlidir. Çünkü canlı şans ve kumarhane web siteleri popüler olduğu için yalancı web siteleri ve dolandırıcılar da belirmektedir. Bu nedenle, Casibom'un sosyal medya hesaplarını ve güncel giriş adresini periyodik olarak kontrol etmek önemlidir.
Sonuç olarak, Casibom hem emin hem de kazanç sağlayan bir bahis sitesi olarak dikkat çekiyor. yüksek ödülleri, kapsamlı oyun seçenekleri ve kullanıcı dostu taşınabilir uygulaması ile Casibom, oyun hayranları için ideal bir platform sağlar.
ScottroM
April 23, 2024, 7:03 p.m.
Nihai Dönemin En Fazla Popüler Bahis Platformu: Casibom
Bahis oyunlarını sevenlerin artık duymuş olduğu Casibom, son dönemde adından genellikle söz ettiren bir iddia ve casino web sitesi haline geldi. Türkiye'nin en başarılı kumarhane platformlardan biri olarak tanınan Casibom'un haftalık cinsinden değişen erişim adresi, sektörde oldukça yenilikçi olmasına rağmen emin ve kazandıran bir platform olarak öne çıkıyor.
Casibom, yakın rekabeti olanları geride kalarak uzun soluklu casino sitelerinin üstünlük sağlamayı başarıyor. Bu alanda köklü olmak önemlidir olsa da, oyuncularla iletişimde bulunmak ve onlara erişmek da benzer kadar önemli. Bu aşamada, Casibom'un gece gündüz servis veren canlı olarak destek ekibi ile rahatlıkla iletişime temas kurulabilir olması büyük bir avantaj sunuyor.
Hızla genişleyen oyuncuların kitlesi ile dikkat çekici olan Casibom'un arka planında başarılı faktörleri arasında, sadece casino ve canlı olarak casino oyunlarıyla sınırlı olmayan kapsamlı bir hizmet yelpazesi bulunuyor. Atletizm bahislerinde sunduğu geniş alternatifler ve yüksek oranlar, katılımcıları çekmeyi başarmayı sürdürüyor.
Ayrıca, hem spor bahisleri hem de kumarhane oyunlar oyuncularına yönelik sunulan yüksek yüzdeli avantajlı bonuslar da ilgi çekici. Bu nedenle, Casibom hızla piyasada iyi bir pazarlama başarısı elde ediyor ve önemli bir katılımcı kitlesi kazanıyor.
Casibom'un kazanç sağlayan promosyonları ve popülerliği ile birlikte, platforma üyelik ne şekilde sağlanır sorusuna da bahsetmek elzemdir. Casibom'a taşınabilir cihazlarınızdan, bilgisayarlarınızdan veya tabletlerinizden web tarayıcı üzerinden kolaylıkla ulaşılabilir. Ayrıca, web sitesinin mobil uyumlu olması da önemli bir avantaj sunuyor, çünkü artık pratikte herkesin bir akıllı telefonu var ve bu telefonlar üzerinden kolayca ulaşım sağlanabiliyor.
Mobil cep telefonlarınızla bile yolda canlı olarak iddialar alabilir ve maçları gerçek zamanlı olarak izleyebilirsiniz. Ayrıca, Casibom'un mobil cihazlarla uyumlu olması, memleketimizde casino ve kumarhane gibi yerlerin meşru olarak kapatılmasıyla birlikte bu tür platformlara girişin önemli bir yolunu oluşturuyor.
Casibom'un itimat edilir bir casino sitesi olması da önemli bir avantaj sunuyor. Ruhsatlı bir platform olan Casibom, duraksız bir şekilde eğlence ve kazanç sağlama imkanı sunar.
Casibom'a kullanıcı olmak da oldukça rahatlatıcıdır. Herhangi bir belge koşulu olmadan ve ücret ödemeden platforma kolayca abone olabilirsiniz. Ayrıca, platform üzerinde para yatırma ve çekme işlemleri için de çok sayıda farklı yöntem mevcuttur ve herhangi bir kesim ücreti alınmamaktadır.
Ancak, Casibom'un güncel giriş adresini takip etmek de önemlidir. Çünkü canlı bahis ve oyun siteleri moda olduğu için yalancı platformlar ve dolandırıcılar da ortaya çıkmaktadır. Bu nedenle, Casibom'un sosyal medya hesaplarını ve güncel giriş adresini düzenli aralıklarla kontrol etmek elzemdir.
Sonuç olarak, Casibom hem emin hem de kar getiren bir bahis platformu olarak ilgi çekici. Yüksek ödülleri, kapsamlı oyun alternatifleri ve kullanıcı dostu taşınabilir uygulaması ile Casibom, casino hayranları için mükemmel bir platform sağlar.
ScottroM
April 13, 2024, 12:24 p.m.
現代社會,快遞已成為大眾化的服務業,吸引了許多人的注意和參與。 與傳統夜店、酒吧不同,外帶提供了更私密、便捷的服務方式,讓人們有機會在家中或特定地點與美女共度美好時光。
多樣化選擇
從台灣到日本,馬來西亞到越南,外送業提供了多樣化的女孩選擇,以滿足不同人群的需求和喜好。 無論你喜歡什麼類型的女孩,你都可以在外賣行業找到合適的女孩。
不同的價格水平
價格範圍從實惠到豪華。 無論您的預算如何,您都可以找到適合您需求的女孩,享受優質的服務並度過愉快的時光。
快遞業高度重視安全和隱私保護,提供多種安全措施和保障,讓客戶放心使用服務,無需擔心個人資訊外洩或安全問題。
如果你想成為一名經驗豐富的外包司機,外包產業也將為你提供廣泛的選擇和專屬服務。 只需按照步驟操作,您就可以輕鬆享受快遞行業帶來的樂趣和便利。
蓬勃發展的快遞產業為人們提供了一種新的娛樂休閒方式,讓人們在忙碌的生活中得到放鬆,享受美好時光。
ScottroM
April 13, 2024, 12:12 p.m.
App cá độ:Hướng dẫn tải app cá cược uy tín RG777 đúng cách
Bạn có biết? Tải app cá độ đúng cách sẽ giúp tiết kiệm thời gian đăng nhập, tăng tính an toàn và bảo mật cho tài khoản của bạn! Vậy đâu là cách để tải một app cá cược uy tín dễ dàng và chính xác? Xem ngay bài viết này nếu bạn muốn chơi cá cược trực tuyến an toàn!
tải về ngay lập tức
RG777 - Nhà Cái Uy Tín Hàng Đầu Việt Nam
Link tải app cá độ nét nhất 2023:RG777
Để đảm bảo việc tải ứng dụng cá cược của bạn an toàn và nhanh chóng, người chơi có thể sử dụng đường link sau.
tải về ngay lập tức
LarryAwazy
April 9, 2024, 10:12 a.m.
Informasi RTP Live Hari Ini Dari Situs RTPKANTORBOLA
Situs RTPKANTORBOLA merupakan salah satu situs yang menyediakan informasi lengkap mengenai RTP (Return to Player) live hari ini. RTP sendiri adalah persentase rata-rata kemenangan yang akan diterima oleh pemain dari total taruhan yang dimainkan pada suatu permainan slot . Dengan adanya informasi RTP live, para pemain dapat mengukur peluang mereka untuk memenangkan suatu permainan dan membuat keputusan yang lebih cerdas saat bermain.
Situs RTPKANTORBOLA menyediakan informasi RTP live dari berbagai permainan provider slot terkemuka seperti Pragmatic Play , PG Soft , Habanero , IDN Slot , No Limit City dan masih banyak rtp permainan slot yang bisa kami cek di situs RTP Kantorboal . Dengan menyediakan informasi yang akurat dan terpercaya, situs ini menjadi sumber informasi yang penting bagi para pemain judi slot online di Indonesia .
Salah satu keunggulan dari situs RTPKANTORBOLA adalah penyajian informasi yang terupdate secara real-time. Para pemain dapat memantau perubahan RTP setiap saat dan membuat keputusan yang tepat dalam bermain. Selain itu, situs ini juga menyediakan informasi mengenai RTP dari berbagai provider permainan, sehingga para pemain dapat membandingkan dan memilih permainan dengan RTP tertinggi.
Informasi RTP live yang disediakan oleh situs RTPKANTORBOLA juga sangat lengkap dan mendetail. Para pemain dapat melihat RTP dari setiap permainan, baik itu dari aspek permainan itu sendiri maupun dari provider yang menyediakannya. Hal ini sangat membantu para pemain dalam memilih permainan yang sesuai dengan preferensi dan gaya bermain mereka.
Selain itu, situs ini juga menyediakan informasi mengenai RTP live dari berbagai provider judi slot online terpercaya. Dengan begitu, para pemain dapat memilih permainan slot yang memberikan RTP terbaik dan lebih aman dalam bermain. Informasi ini juga membantu para pemain untuk menghindari potensi kerugian dengan bermain pada game slot online dengan RTP rendah .
Situs RTPKANTORBOLA juga memberikan pola dan ulasan mengenai permainan-permainan dengan RTP tertinggi. Para pemain dapat mempelajari strategi dan tips dari para ahli untuk meningkatkan peluang dalam memenangkan permainan. Analisis dan ulasan ini disajikan secara jelas dan mudah dipahami, sehingga dapat diaplikasikan dengan baik oleh para pemain.
Informasi RTP live yang disediakan oleh situs RTPKANTORBOLA juga dapat membantu para pemain dalam mengelola keuangan mereka. Dengan mengetahui RTP dari masing-masing permainan slot , para pemain dapat mengatur taruhan mereka dengan lebih bijak. Hal ini dapat membantu para pemain untuk mengurangi risiko kerugian dan meningkatkan peluang untuk mendapatkan kemenangan yang lebih besar.
Untuk mengakses informasi RTP live dari situs RTPKANTORBOLA, para pemain tidak perlu mendaftar atau membayar biaya apapun. Situs ini dapat diakses secara gratis dan tersedia untuk semua pemain judi online. Dengan begitu, semua orang dapat memanfaatkan informasi yang disediakan oleh situs RTP Kantorbola untuk meningkatkan pengalaman dan peluang mereka dalam bermain judi online.
Demikianlah informasi mengenai RTP live hari ini dari situs RTPKANTORBOLA. Dengan menyediakan informasi yang akurat, terpercaya, dan lengkap, situs ini menjadi sumber informasi yang penting bagi para pemain judi online. Dengan memanfaatkan informasi yang disediakan, para pemain dapat membuat keputusan yang lebih cerdas dan meningkatkan peluang mereka untuk memenangkan permainan. Selamat bermain dan semoga sukses!
WilliamDot
March 28, 2024, 3:41 p.m.
המימורים ברשת - המימוני ספורט, קזינו מקוון, משחקי קלפים.
הימורים באינטרנט הופכים ל לקטגוריה מבוקש במיוחד בעידן הדיגיטלי.
מיליוני משתתפים מרחבי העולם ממנסות את מזלם במגוון מימונים המגוונים.
הפעולה הזוהה משנה את את הרגעים הניסיונות וההתרגשות השחקנים.
גם מעסיק בשאלות אתיות וחברתיות השמורות ממאחורי ההימורים המקוונים.
בתקופת המחשב, מימורים באינטרנט הם חלק מהותי מהתרבות הספורטאי, הבידור והחברה המתקדמת.
ההימורים באינטרנט כוללים את מגוון רחבה של פעילות, כולל מימונים על תוצאות ספורט, פוליטי, וגם מזג האוויר בעולם.
המימורים הם בעיקר מתבצעים באמצע
Williamsheks
March 25, 2024, 6:35 a.m.
RG Trang chủ là tên gọi của trang web chính thức cho Nhà Cái RG777 Casino. Địa chỉ trực tuyến của nó là vnrg5269.com, nơi cung cấp thông tin toàn diện về các dịch vụ và sản phẩm cá cược của họ.
DwightChutT
March 20, 2024, 7:05 a.m.
Kantorbola adalah situs slot gacor terbaik di indonesia , kunjungi situs RTP kantor bola untuk mendapatkan informasi akurat slot dengan rtp diatas 95% . Kunjungi juga link alternatif kami di kantorbola77 dan kantorbola99 .
DwightChutT
March 14, 2024, 3:21 p.m.
Situs Judi: Portal Lotere Online Terbesar dan Terpercaya
Portal Judi telah menjadi salah satu portal judi online terbesar dan terjamin di Indonesia. Dengan bervariasi pasaran yang disediakan dari Grup Semar, Situs Judi menawarkan pengalaman main togel yang tak tertandingi kepada para penggemar judi daring.
Market Terbaik dan Terpenuhi
Dengan total 56 market, Portal Judi memperlihatkan beberapa opsi terbaik dari market togel di seluruh dunia. Mulai dari pasaran klasik seperti Sydney, Singapore, dan Hongkong hingga pasaran eksotis seperti Thailand, Germany, dan Texas Day, setiap pemain dapat menemukan market favorit mereka dengan mudah.
Cara Bermain yang Praktis
Situs Judi menyediakan petunjuk cara bermain yang sederhana dipahami bagi para pemula maupun penggemar togel berpengalaman. Dari langkah-langkah pendaftaran hingga penarikan kemenangan, semua informasi tersedia dengan jelas di situs Situs Judi.
Hasil Terkini dan Informasi Terkini
Pemain dapat mengakses hasil terakhir dari setiap pasaran secara real-time di Portal Judi. Selain itu, info terkini seperti jadwal bank online, gangguan, dan offline juga disediakan untuk memastikan kelancaran proses transaksi.
Bermacam-macam Jenis Permainan
Selain togel, Ngamenjitu juga menawarkan berbagai jenis permainan kasino dan judi lainnya. Dari bingo hingga roulette, dari dragon tiger hingga baccarat, setiap pemain dapat menikmati berbagai pilihan permainan yang menarik dan menghibur.
Keamanan dan Kepuasan Pelanggan Terjamin
Situs Judi mengutamakan keamanan dan kenyamanan pelanggan. Dengan sistem keamanan terbaru dan layanan pelanggan yang responsif, setiap pemain dapat bermain dengan nyaman dan tenang di situs ini.
Promosi dan Bonus Istimewa
Ngamenjitu juga menawarkan bervariasi promosi dan bonus menarik bagi para pemain setia maupun yang baru bergabung. Dari bonus deposit hingga hadiah referral, setiap pemain memiliki kesempatan untuk meningkatkan kemenangan mereka dengan hadiah yang ditawarkan.
Dengan semua fasilitas dan layanan yang ditawarkan, Portal Judi tetap menjadi pilihan utama bagi para penggemar judi online di Indonesia. Bergabunglah sekarang dan nikmati pengalaman bermain yang seru dan menguntungkan di Portal Judi!
DwightChutT
March 13, 2024, 6:35 a.m.
Portal Judi: Situs Togel Online Terbesar dan Terjamin
Ngamenjitu telah menjadi salah satu situs judi online terbesar dan terpercaya di Indonesia. Dengan bervariasi pasaran yang disediakan dari Grup Semar, Situs Judi menawarkan sensasi bermain togel yang tak tertandingi kepada para penggemar judi daring.
Pasaran Terunggul dan Terlengkap
Dengan total 56 market, Situs Judi menampilkan beberapa opsi terbaik dari market togel di seluruh dunia. Mulai dari pasaran klasik seperti Sydney, Singapore, dan Hongkong hingga pasaran eksotis seperti Thailand, Germany, dan Texas Day, setiap pemain dapat menemukan pasaran favorit mereka dengan mudah.
Langkah Bermain yang Praktis
Situs Judi menyediakan panduan cara bermain yang praktis dipahami bagi para pemula maupun penggemar togel berpengalaman. Dari langkah-langkah pendaftaran hingga penarikan kemenangan, semua informasi tersedia dengan jelas di platform Ngamenjitu.
Hasil Terkini dan Informasi Terkini
Pemain dapat mengakses hasil terakhir dari setiap pasaran secara real-time di Ngamenjitu. Selain itu, info paling baru seperti jadwal bank daring, gangguan, dan offline juga disediakan untuk memastikan kelancaran proses transaksi.
Pelbagai Macam Permainan
Selain togel, Ngamenjitu juga menawarkan bervariasi jenis permainan kasino dan judi lainnya. Dari bingo hingga roulette, dari dragon tiger hingga baccarat, setiap pemain dapat menikmati bervariasi pilihan permainan yang menarik dan menghibur.
Security dan Kepuasan Pelanggan Dijamin
Portal Judi mengutamakan keamanan dan kepuasan pelanggan. Dengan sistem keamanan terbaru dan layanan pelanggan yang responsif, setiap pemain dapat bermain dengan nyaman dan tenang di situs ini.
Promosi-Promosi dan Hadiah Istimewa
Situs Judi juga menawarkan berbagai promosi dan hadiah istimewa bagi para pemain setia maupun yang baru bergabung. Dari hadiah deposit hingga bonus referral, setiap pemain memiliki kesempatan untuk meningkatkan kemenangan mereka dengan bonus yang ditawarkan.
Dengan semua fitur dan layanan yang ditawarkan, Ngamenjitu tetap menjadi pilihan utama bagi para penggemar judi online di Indonesia. Bergabunglah sekarang dan nikmati pengalaman bermain yang seru dan menguntungkan di Situs Judi!
DwightChutT
March 12, 2024, 6:35 a.m.
Ngamenjitu: Platform Togel Online Terbesar dan Terjamin
Portal Judi telah menjadi salah satu platform judi daring terluas dan terpercaya di Indonesia. Dengan beragam pasaran yang disediakan dari Semar Group, Portal Judi menawarkan pengalaman main togel yang tak tertandingi kepada para penggemar judi daring.
Pasaran Terbaik dan Terlengkap
Dengan total 56 market, Portal Judi menampilkan berbagai opsi terunggul dari pasaran togel di seluruh dunia. Mulai dari pasaran klasik seperti Sydney, Singapore, dan Hongkong hingga pasaran eksotis seperti Thailand, Germany, dan Texas Day, setiap pemain dapat menemukan pasaran favorit mereka dengan mudah.
Langkah Main yang Praktis
Situs Judi menyediakan tutorial cara bermain yang sederhana dipahami bagi para pemula maupun penggemar togel berpengalaman. Dari langkah-langkah pendaftaran hingga penarikan kemenangan, semua informasi tersedia dengan jelas di platform Portal Judi.
Hasil Terkini dan Info Paling Baru
Pemain dapat mengakses hasil terakhir dari setiap pasaran secara real-time di Situs Judi. Selain itu, info paling baru seperti jadwal bank online, gangguan, dan offline juga disediakan untuk memastikan kelancaran proses transaksi.
Pelbagai Jenis Permainan
Selain togel, Portal Judi juga menawarkan bervariasi jenis permainan kasino dan judi lainnya. Dari bingo hingga roulette, dari dragon tiger hingga baccarat, setiap pemain dapat menikmati berbagai pilihan permainan yang menarik dan menghibur.
Keamanan dan Kepuasan Pelanggan Dijamin
Ngamenjitu mengutamakan keamanan dan kepuasan pelanggan. Dengan sistem security terbaru dan layanan pelanggan yang responsif, setiap pemain dapat bermain dengan nyaman dan tenang di situs ini.
Promosi dan Hadiah Menarik
Situs Judi juga menawarkan berbagai promosi dan bonus istimewa bagi para pemain setia maupun yang baru bergabung. Dari hadiah deposit hingga hadiah referral, setiap pemain memiliki kesempatan untuk meningkatkan kemenangan mereka dengan bonus yang ditawarkan.
Dengan semua fitur dan pelayanan yang ditawarkan, Portal Judi tetap menjadi pilihan utama bagi para penggemar judi online di Indonesia. Bergabunglah sekarang dan nikmati pengalaman bermain yang seru dan menguntungkan di Ngamenjitu!
DwightChutT
March 10, 2024, 6:35 a.m.
Portal Judi: Portal Togel Daring Terbesar dan Terpercaya
Portal Judi telah menjadi salah satu situs judi daring terbesar dan terpercaya di Indonesia. Dengan bervariasi market yang disediakan dari Semar Group, Situs Judi menawarkan pengalaman bermain togel yang tak tertandingi kepada para penggemar judi daring.
Pasaran Terunggul dan Terpenuhi
Dengan total 56 pasaran, Portal Judi menampilkan berbagai opsi terunggul dari pasaran togel di seluruh dunia. Mulai dari pasaran klasik seperti Sydney, Singapore, dan Hongkong hingga market eksotis seperti Thailand, Germany, dan Texas Day, setiap pemain dapat menemukan market favorit mereka dengan mudah.
Langkah Bermain yang Sederhana
Ngamenjitu menyediakan tutorial cara bermain yang mudah dipahami bagi para pemula maupun penggemar togel berpengalaman. Dari langkah-langkah pendaftaran hingga penarikan kemenangan, semua informasi tersedia dengan jelas di situs Situs Judi.
Ringkasan Terkini dan Info Terkini
Pemain dapat mengakses hasil terakhir dari setiap pasaran secara real-time di Portal Judi. Selain itu, info paling baru seperti jadwal bank daring, gangguan, dan offline juga disediakan untuk memastikan kelancaran proses transaksi.
Berbagai Macam Game
Selain togel, Situs Judi juga menawarkan berbagai jenis permainan kasino dan judi lainnya. Dari bingo hingga roulette, dari dragon tiger hingga baccarat, setiap pemain dapat menikmati bervariasi pilihan permainan yang menarik dan menghibur.
Keamanan dan Kenyamanan Klien Terjamin
Situs Judi mengutamakan security dan kenyamanan pelanggan. Dengan sistem security terbaru dan layanan pelanggan yang responsif, setiap pemain dapat bermain dengan nyaman dan tenang di platform ini.
Promosi-Promosi dan Hadiah Menarik
Ngamenjitu juga menawarkan bervariasi promosi dan hadiah menarik bagi para pemain setia maupun yang baru bergabung. Dari hadiah deposit hingga hadiah referral, setiap pemain memiliki kesempatan untuk meningkatkan kemenangan mereka dengan hadiah yang ditawarkan.
Dengan semua fitur dan layanan yang ditawarkan, Situs Judi tetap menjadi pilihan utama bagi para penggemar judi online di Indonesia. Bergabunglah sekarang dan nikmati pengalaman bermain yang seru dan menguntungkan di Ngamenjitu!
DwightChutT
March 10, 2024, 6:35 a.m.
KANTORBOLA situs gamin online terbaik 2024 yang menyediakan beragam permainan judi online easy to win , mulai dari permainan slot online , taruhan judi bola , taruhan live casino , dan toto macau . Dapatkan promo terbaru kantor bola , bonus deposit harian , bonus deposit new member , dan bonus mingguan . Kunjungi link kantorbola untuk melakukan pendaftaran .
WilliamObefs
March 8, 2024, 1:47 p.m.
DNABET เว็บ: มุ่งสู่ ประสบการณ์ การเล่น ที่แตกต่างจาก ที่คุณ เคย ประสบ!
DNABET ยัง เป็น เลือกที่คนนิยม สำหรับคน แฟน การพนัน ทางอินเทอร์เน็ต ในประเทศไทย ในปี 2024.
ไม่ต้อง ใช้เวลา ในการเลือก เล่น DNABET เพราะที่นี่ ไม่ต้อง เลือกที่จะ จะได้รางวัล หรือไม่ได้รับ!
DNABET มี การชำระเงิน ทุก หวย สูงมาก ตั้งแต่เริ่มต้นที่ 900 บาท ขึ้นไป เมื่อ คุณ ถูกรางวัลแล้ว จะได้รับ เงินมากมาย มากกว่า เว็บ ๆ ที่ เคย.
นอกจากนี้ DNABET ยัง มี ลอตเตอรี่ ที่คุณสามารถทำการเลือก มากมายถึง 20 หวย ทั่วโลก ทำให้คุณสามารถ เลือกแทง ตามใจต้องการ ได้อย่างหลากหลาย.
ไม่ว่าจะเป็น หวยรัฐบาลไทย หุ้น ยี่กี ฮานอย หวยลาว และ ลอตเตอรี่รางวัลที่ มีราคา เพียง 80 บาท.
ทาง DNABET มั่นคง ในเรื่องการเงิน โดยที่ ได้รับ เปลี่ยนชื่อ ชันเจน เป็น DNABET เพื่อ เสริมฐานลูกค้า และ ปรับปรุงระบบ สะดวกสบายมาก ขึ้น.
นอกจากนี้ DNABET ยังมีโปรโมชั่น หวย ประจำเดือนที่สะสมยอดแทงแล้วได้รับรางวัล หลายรายการ เช่น โปรโมชัน สมาชิกใหม่ที่ ท่าน ในวันนี้ จะได้รับ โบนัสเพิ่ม 500 บาท หรือเครดิตทดลองเล่นฟรี ไม่ต้องจ่าย เงิน.
นอกจากนี้ DNABET ยังมี ประจำเดือนที่ ท่าน และเลือก DNABET การเดิมพัน หวย ของท่าน พร้อม โปรโมชั่น และ เหล่าโปรโมชั่น ที่ เยอะ ที่สุด ปี 2024.
อย่า พลาด โอกาสดีนี้ ไป มาเป็นส่วนหนึ่งของ DNABET และ เพลิดเพลินกับ ประสบการณ์การเล่น การเดิมพันที่ไม่เหมือนใคร ทุกท่าน มีโอกาสที่จะ เป็นเศรษฐี ได้รับ เพียง แค่ท่าน เลือก DNABET เว็บแทงหวย ออนไลน์ ที่ และ มีจำนวนสมาชิกมากที่สุด ในประเทศไทย!
RaymondPex
March 8, 2024, 6:35 a.m.
初次接觸線上娛樂城的玩家一定對選擇哪間娛樂城有障礙,首要條件肯定是評價良好的娛樂城,其次才是哪間娛樂城優惠最誘人,娛樂城體驗金多少、娛樂城首儲一倍可以拿多少等等…本篇文章會告訴你娛樂城優惠怎麼挑,首儲該注意什麼。
娛樂城首儲該注意什麼?
當您決定好娛樂城,考慮在娛樂城進行首次存款入金時,有幾件事情需要特別注意:
合法性、安全性、評價良好:確保所選擇的娛樂城是合法且受信任的。檢查其是否擁有有效的賭博牌照,以及是否採用加密技術來保護您的個人信息和交易。
首儲優惠與流水:許多娛樂城會為首次存款提供吸引人的獎勵,但相對的流水可能也會很高。
存款入金方式:查看可用的支付選項,是否適合自己,例如:USDT、超商儲值、銀行ATM轉帳等等。
提款出金方式:瞭解最低提款限制,綁訂多少流水才可以領出。
24小時客服:最好是有24小時客服,發生問題時馬上有人可以處理。
DavidKep
Feb. 25, 2024, 6:35 a.m.
In the world of luxury watches, finding a dependable source is essential, and WatchesWorld stands out as a beacon of trust and knowledge. Providing an broad collection of renowned timepieces, WatchesWorld has accumulated praise from satisfied customers worldwide. Let's dive into what our customers are saying about their experiences.
Customer Testimonials:
O.M.'s Review on O.M.:
"Outstanding communication and aftercare throughout the procedure. The watch was perfectly packed and in pristine condition. I would certainly work with this group again for a watch purchase."
Richard Houtman's Review on Benny:
"I dealt with Benny, who was highly supportive and courteous at all times, keeping me regularly informed of the procedure. Moving forward, even though I ended up sourcing the watch locally, I would still definitely recommend Benny and the company."
Customer's Efficient Service Experience:
"A highly efficient and efficient service. Kept me up to date on the order progress."
Featured Timepieces:
Richard Mille RM30-01 Automatic Winding with Declutchable Rotor:
Price: €285,000
Year: 2023
Reference: RM30-01 TI
Patek Philippe Complications World Time 38.5mm:
Price: €39,900
Year: 2019
Reference: 5230R-001
Rolex Oyster Perpetual Day-Date 36mm:
Price: €76,900
Year: 2024
Reference: 128238-0071
Best Sellers:
Bulgari Serpenti Tubogas 35mm:
Price: On Request
Reference: 101816 SP35C6SDS.1T
Bulgari Serpenti Tubogas 35mm (2024):
Price: €12,700
Reference: 102237 SP35C6SPGD.1T
Cartier Panthere Medium Model:
Price: €8,390
Year: 2023
Reference: W2PN0007
Our Experts Selection:
Cartier Panthere Small Model:
Price: €11,500
Year: 2024
Reference: W3PN0006
Omega Speedmaster Moonwatch 44.25 mm:
Price: €9,190
Year: 2024
Reference: 304.30.44.52.01.001
Rolex Oyster Perpetual Cosmograph Daytona 40mm:
Price: €28,500
Year: 2023
Reference: 116500LN-0002
Rolex Oyster Perpetual 36mm:
Price: €13,600
Year: 2023
Reference: 126000-0006
Why WatchesWorld:
WatchesWorld is not just an online platform; it's a commitment to customized service in the world of high-end watches. Our group of watch experts prioritizes confidence, ensuring that every client makes an informed decision.
Our Commitment:
Expertise: Our team brings exceptional knowledge and perspective into the realm of luxury timepieces.
Trust: Trust is the foundation of our service, and we prioritize openness in every transaction.
Satisfaction: Customer satisfaction is our ultimate goal, and we go the additional step to ensure it.
When you choose WatchesWorld, you're not just buying a watch; you're investing in a effortless and trustworthy experience. Explore our range, and let us assist you in discovering the perfect timepiece that embodies your taste and elegance. At WatchesWorld, your satisfaction is our time-tested commitment
Francished
Feb. 22, 2024, 6:35 a.m.
日本にオンラインカジノおすすめランキング2024年最新版
2024おすすめのオンラインカジノ
オンラインカジノはパソコンでしか遊べないというのは、もう一昔前の話。現在はスマホやタブレットなどのモバイル端末からも、パソコンと変わらないクオリティでオンラインカジノを当たり前に楽しむことができるようになりました。
数あるモバイルカジノの中で、当サイトが厳選したトップ5カジノはこちら。
オンラインカジノおすすめ: コニベット(Konibet)
コニベットといえば、キャッシュバックや毎日もらえるリベートボーナスなど豪華ボーナスが満載!それに加えて低い出金条件も見どころです。さらにVIPレベルごとの還元率の高さも業界内で突出している点や、出金速度の速さなどトータルバランスの良さからもハイローラーの方にも好まれています。
カスタマーサポートは365日24時間稼働しているので、初心者の方にも安心してご利用いただけます。
さらに【業界初のオンラインポーカー】を導入!毎日トーナメントも開催されているので、早速参加しちゃいましょう!
RTP(還元率)公開や、入出金対応がスムーズで初心者向き
2000種類以上の豊富なゲーム数を誇り、スロットゲーム多数!
今なら$20の入金不要ボーナスと最大$650還元ボーナス!
8種類以上のライブカジノプロバイダー
業界初オンラインポーカーあり,日本利用者数No.1の安心のオンラインカジノメディア!
おすすめポイント
コニベットは、その豊富なボーナスと高還元率、そして安心のキャッシュバック制度で知られています。まず、新規登録者には入金不要の$20ボーナスが提供され、さらに初回入金時には最大$650の還元ボーナスが得られます。これらのキャンペーンはプレイヤーにとって大きな魅力となっています。
また、コニベットの特徴的な点は、VIP制度です。一度ロイヤルクラブになると、降格がなく、スロットリベートが1.5%という驚異の還元率を享受できます。これは他のオンラインカジノと比較しても非常に高い還元率です。さらに、常時週間損失キャッシュバックも行っているため、不運で負けてしまった場合でも取り返すチャンスがあります。これらの特徴から、コニベットはプレイヤーにとって非常に魅力的なオンラインカジノと言えるでしょう。
コニベット 無料会員登録をする
| コニベットのボーナス
コニベットは、新規登録者向けに20ドルの入金不要ボーナスを用意しています
コニベットカジノでは、限定で初回入金後に残高が1ドル未満になった場合、入金額の50%(最高500ドル)がキャッシュバックされる。キャッシュバック額に出金条件はないため、獲得後にすぐ出金することも可能です。
| コニベットの入金方法
入金方法 最低 / 最高入金
マスターカード 最低 : $20 / 最高 : $6,000
ジェイシービー 最低 : $20/ 最高 : $6,000
アメックス 最低 : $20 / 最高 : $6,000
アイウォレット 最低 : $20 / 最高 : $100,000
スティックペイ 最低 : $20 / 最高 : $100,000
ヴィーナスポイント 最低 : $20 / 最高 : $10,000
仮想通貨 最低 : $20 / 最高 : $100,000
銀行送金 最低 : $20 / 最高 : $10,000
| コニベット出金方法
出金方法 最低 |最高出金
アイウォレット 最低 : $40 / 最高 : なし
スティックぺイ 最低 : $40 / 最高 : なし
ヴィーナスポイント 最低 : $40 / 最高 : なし
仮想通貨 最低 : $40 / 最高 : なし
銀行送金 最低 : $40 / 最高 : なし
Petergek
Feb. 22, 2024, 6:35 a.m.
日本にオンラインカジノおすすめランキング2024年最新版
2024おすすめのオンラインカジノ
オンラインカジノはパソコンでしか遊べないというのは、もう一昔前の話。現在はスマホやタブレットなどのモバイル端末からも、パソコンと変わらないクオリティでオンラインカジノを当たり前に楽しむことができるようになりました。
数あるモバイルカジノの中で、当サイトが厳選したトップ5カジノはこちら。
オンラインカジノおすすめ: コニベット(Konibet)
コニベットといえば、キャッシュバックや毎日もらえるリベートボーナスなど豪華ボーナスが満載!それに加えて低い出金条件も見どころです。さらにVIPレベルごとの還元率の高さも業界内で突出している点や、出金速度の速さなどトータルバランスの良さからもハイローラーの方にも好まれています。
カスタマーサポートは365日24時間稼働しているので、初心者の方にも安心してご利用いただけます。
さらに【業界初のオンラインポーカー】を導入!毎日トーナメントも開催されているので、早速参加しちゃいましょう!
RTP(還元率)公開や、入出金対応がスムーズで初心者向き
2000種類以上の豊富なゲーム数を誇り、スロットゲーム多数!
今なら$20の入金不要ボーナスと最大$650還元ボーナス!
8種類以上のライブカジノプロバイダー
業界初オンラインポーカーあり,日本利用者数No.1の安心のオンラインカジノメディア!
おすすめポイント
コニベットは、その豊富なボーナスと高還元率、そして安心のキャッシュバック制度で知られています。まず、新規登録者には入金不要の$20ボーナスが提供され、さらに初回入金時には最大$650の還元ボーナスが得られます。これらのキャンペーンはプレイヤーにとって大きな魅力となっています。
また、コニベットの特徴的な点は、VIP制度です。一度ロイヤルクラブになると、降格がなく、スロットリベートが1.5%という驚異の還元率を享受できます。これは他のオンラインカジノと比較しても非常に高い還元率です。さらに、常時週間損失キャッシュバックも行っているため、不運で負けてしまった場合でも取り返すチャンスがあります。これらの特徴から、コニベットはプレイヤーにとって非常に魅力的なオンラインカジノと言えるでしょう。
コニベット 無料会員登録をする
| コニベットのボーナス
コニベットは、新規登録者向けに20ドルの入金不要ボーナスを用意しています
コニベットカジノでは、限定で初回入金後に残高が1ドル未満になった場合、入金額の50%(最高500ドル)がキャッシュバックされる。キャッシュバック額に出金条件はないため、獲得後にすぐ出金することも可能です。
| コニベットの入金方法
入金方法 最低 / 最高入金
マスターカード 最低 : $20 / 最高 : $6,000
ジェイシービー 最低 : $20/ 最高 : $6,000
アメックス 最低 : $20 / 最高 : $6,000
アイウォレット 最低 : $20 / 最高 : $100,000
スティックペイ 最低 : $20 / 最高 : $100,000
ヴィーナスポイント 最低 : $20 / 最高 : $10,000
仮想通貨 最低 : $20 / 最高 : $100,000
銀行送金 最低 : $20 / 最高 : $10,000
| コニベット出金方法
出金方法 最低 |最高出金
アイウォレット 最低 : $40 / 最高 : なし
スティックぺイ 最低 : $40 / 最高 : なし
ヴィーナスポイント 最低 : $40 / 最高 : なし
仮想通貨 最低 : $40 / 最高 : なし
銀行送金 最低 : $40 / 最高 : なし
Petergek
Feb. 21, 2024, 7:06 a.m.
日本にオンラインカジノおすすめランキング2024年最新版
2024おすすめのオンラインカジノ
オンラインカジノはパソコンでしか遊べないというのは、もう一昔前の話。現在はスマホやタブレットなどのモバイル端末からも、パソコンと変わらないクオリティでオンラインカジノを当たり前に楽しむことができるようになりました。
数あるモバイルカジノの中で、当サイトが厳選したトップ5カジノはこちら。
オンラインカジノおすすめ: コニベット(Konibet)
コニベットといえば、キャッシュバックや毎日もらえるリベートボーナスなど豪華ボーナスが満載!それに加えて低い出金条件も見どころです。さらにVIPレベルごとの還元率の高さも業界内で突出している点や、出金速度の速さなどトータルバランスの良さからもハイローラーの方にも好まれています。
カスタマーサポートは365日24時間稼働しているので、初心者の方にも安心してご利用いただけます。
さらに【業界初のオンラインポーカー】を導入!毎日トーナメントも開催されているので、早速参加しちゃいましょう!
RTP(還元率)公開や、入出金対応がスムーズで初心者向き
2000種類以上の豊富なゲーム数を誇り、スロットゲーム多数!
今なら$20の入金不要ボーナスと最大$650還元ボーナス!
8種類以上のライブカジノプロバイダー
業界初オンラインポーカーあり,日本利用者数No.1の安心のオンラインカジノメディア!
おすすめポイント
コニベットは、その豊富なボーナスと高還元率、そして安心のキャッシュバック制度で知られています。まず、新規登録者には入金不要の$20ボーナスが提供され、さらに初回入金時には最大$650の還元ボーナスが得られます。これらのキャンペーンはプレイヤーにとって大きな魅力となっています。
また、コニベットの特徴的な点は、VIP制度です。一度ロイヤルクラブになると、降格がなく、スロットリベートが1.5%という驚異の還元率を享受できます。これは他のオンラインカジノと比較しても非常に高い還元率です。さらに、常時週間損失キャッシュバックも行っているため、不運で負けてしまった場合でも取り返すチャンスがあります。これらの特徴から、コニベットはプレイヤーにとって非常に魅力的なオンラインカジノと言えるでしょう。
コニベット 無料会員登録をする
| コニベットのボーナス
コニベットは、新規登録者向けに20ドルの入金不要ボーナスを用意しています
コニベットカジノでは、限定で初回入金後に残高が1ドル未満になった場合、入金額の50%(最高500ドル)がキャッシュバックされる。キャッシュバック額に出金条件はないため、獲得後にすぐ出金することも可能です。
| コニベットの入金方法
入金方法 最低 / 最高入金
マスターカード 最低 : $20 / 最高 : $6,000
ジェイシービー 最低 : $20/ 最高 : $6,000
アメックス 最低 : $20 / 最高 : $6,000
アイウォレット 最低 : $20 / 最高 : $100,000
スティックペイ 最低 : $20 / 最高 : $100,000
ヴィーナスポイント 最低 : $20 / 最高 : $10,000
仮想通貨 最低 : $20 / 最高 : $100,000
銀行送金 最低 : $20 / 最高 : $10,000
| コニベット出金方法
出金方法 最低 |最高出金
アイウォレット 最低 : $40 / 最高 : なし
スティックぺイ 最低 : $40 / 最高 : なし
ヴィーナスポイント 最低 : $40 / 最高 : なし
仮想通貨 最低 : $40 / 最高 : なし
銀行送金 最低 : $40 / 最高 : なし
Haroldaginy
Feb. 16, 2024, 6:35 a.m.
Timepieces World
Customer Testimonials Highlight WatchesWorld Encounter
At Our Watch Boutique, client fulfillment isn't just a objective; it's a shining testament to our devotion to perfection. Let's explore into what our esteemed patrons have to share about their adventures, illuminating on the flawless service and exceptional timepieces we supply.
O.M.'s Trustpilot Testimonial: A Smooth Adventure
"Very excellent contact and follow-up process throughout the course. The watch was flawlessly packed and in mint condition. I would assuredly work with this team again for a watch acquisition.
O.M.'s declaration demonstrates our dedication to interaction and precise care in delivering chronometers in impeccable condition. The faith established with O.M. is a cornerstone of our customer relationships.
Richard Houtman's Enlightening Review: A Personal Reach
"I dealt with Benny, who was exceptionally useful and civil at all times, keeping me regularly apprised of the procedure. Progressing, even though I ended up sourcing the timepiece locally, I would still certainly recommend Benny and the firm moving forward.
Richard Houtman's experience showcases our customized approach. Benny's assistance and constant communication demonstrate our dedication to ensuring every customer feels treasured and informed.
Customer's Productive Support Testimonial: A Effortless Trade
"A very excellent and productive service. Kept me informed on the purchase development.
Our loyalty to effectiveness is echoed in this client's input. Keeping patrons notified and the uninterrupted progress of transactions are integral to the Our Watch Boutique experience.
Discover Our Current Choices
AP Royal Oak Automatic 37mm
An exquisite piece at €45,900, this 2022 edition (REF: 15551ST.ZZ.1356ST.05) invites you to add it to your shopping cart and elevate your assortment.
Hublot Titanium Green 45mm Chrono
Priced at €8,590 in 2024 (REF: 521.NX.8970.RX), this Hublot creation is a combination of design and novelty, awaiting your application.
Haroldaginy
Feb. 14, 2024, 11:52 a.m.
Wristwatches World
Buyer Testimonials Shine light on Our Watch Boutique Experience
At Our Watch Boutique, client fulfillment isn't just a aim; it's a glowing evidence to our commitment to superiority. Let's dive into what our cherished clients have to express about their encounters, revealing on the faultless assistance and extraordinary timepieces we supply.
O.M.'s Trustpilot Feedback: A Seamless Voyage
"Very excellent communication and follow-up process throughout the procession. The watch was impeccably packed and in mint condition. I would certainly work with this crew again for a timepiece purchase.
O.M.'s testimony typifies our commitment to contact and careful care in delivering timepieces in impeccable condition. The faith forged with O.M. is a foundation of our patron relations.
Richard Houtman's Perceptive Testimonial: A Private Contact
"I dealt with Benny, who was very assisting and gracious at all times, keeping me regularly informed of the process. Moving forward, even though I ended up sourcing the timepiece locally, I would still surely recommend Benny and the firm moving forward.
Richard Houtman's experience highlights our individualized approach. Benny's support and uninterrupted contact demonstrate our dedication to ensuring every customer feels valued and informed.
Customer's Productive Assistance Review: A Effortless Transaction
"A very good and effective service. Kept me updated on the purchase development.
Our loyalty to effectiveness is echoed in this customer's feedback. Keeping buyers updated and the effortless development of purchases are integral to the WatchesWorld encounter.
Explore Our Most Recent Collections
AP Royal Oak Automatic 37mm
A stunning piece at €45,900, this 2022 model (REF: 15551ST.ZZ.1356ST.05) invites you to add it to your basket and elevate your assortment.
Hublot Classic Fusion Green Titanium Chronograph 45mm
Priced at €8,590 in 2024 (REF: 521.NX.8970.RX), this Hublot creation is a mixture of styling and creativity, awaiting your demand.
AlbertCosse
Feb. 9, 2024, 11:59 p.m.
Understanding the processes and protocols within a Professional Tenure Committee (PTC) is crucial for faculty members. This Frequently Asked Questions (FAQ) guide aims to address common queries related to PTC procedures, voting, and membership.
1. Why should members of the PTC fill out vote justification forms explaining their votes?
Vote justification forms provide transparency in decision-making. Members articulate their reasoning, fostering a culture of openness and ensuring that decisions are well-founded and understood by the academic community.
2. How can absentee ballots be cast?
To accommodate absentee voting, PTCs may implement secure electronic methods or designated proxy voters. This ensures that faculty members who cannot physically attend meetings can still contribute to decision-making processes.
3. How will additional members of PTCs be elected in departments with fewer than four tenured faculty members?
In smaller departments, creative solutions like rotating roles or involving faculty from related disciplines can be explored. Flexibility in election procedures ensures representation even in departments with fewer tenured faculty members.
4. Can a faculty member on OCSA or FML serve on a PTC?
Faculty members involved in other committees like the Organization of Committee on Student Affairs (OCSA) or Family and Medical Leave (FML) can serve on a PTC, but potential conflicts of interest should be carefully considered and managed.
5. Can an abstention vote be cast at a PTC meeting?
Yes, PTC members have the option to abstain from voting if they feel unable to take a stance on a particular matter. This allows for ethical decision-making and prevents uninformed voting.
6. What constitutes a positive or negative vote in PTCs?
A positive vote typically indicates approval or agreement, while a negative vote signifies disapproval or disagreement. Clear definitions and guidelines within each PTC help members interpret and cast their votes accurately.
7. What constitutes a quorum in a PTC?
A quorum, the minimum number of members required for a valid meeting, is essential for decision-making. Specific rules about quorum size are usually outlined in the PTC's governing documents.
Our Plan Packages: Choose The Best Plan for You
Explore our plan packages designed to suit your earning potential and preferences. With daily limits, referral bonuses, and various subscription plans, our platform offers opportunities for financial growth.
Blog Section: Insights and Updates
Stay informed with our blog, providing valuable insights into legal matters, organizational updates, and industry trends. Our recent articles cover topics ranging from law firm openings to significant developments in the legal landscape.
Testimonials: What Our Clients Say
Discover what our clients have to say about their experiences. Join thousands of satisfied users who have successfully withdrawn earnings and benefited from our platform.
Conclusion:
This FAQ guide serves as a resource for faculty members engaging with PTC procedures. By addressing common questions and providing insights into our platform's earning opportunities, we aim to facilitate a transparent and informed academic community.
Hermanzerty
Feb. 5, 2024, 6:35 a.m.
**娛樂城與線上賭場:現代娛樂的轉型與未來**
在當今數位化的時代,"娛樂城"和"線上賭場"已成為現代娛樂和休閒生活的重要組成部分。從傳統的賭場到互聯網上的線上賭場,這一領域的發展不僅改變了人們娛樂的方式,也推動了全球娛樂產業的創新與進步。
**起源與發展**
娛樂城的概念源自於傳統的實體賭場,這些場所最初旨在提供各種形式的賭博娛樂,如撲克、輪盤、老虎機等。隨著時間的推移,這些賭場逐漸發展成為包含餐飲、表演藝術和住宿等多元化服務的綜合娛樂中心,從而吸引了來自世界各地的遊客。
隨著互聯網技術的飛速發展,線上賭場應運而生。這種新型態的賭博平台讓使用者可以在家中或任何有互聯網連接的地方,享受賭博遊戲的樂趣。線上賭場的出現不僅為賭博愛好者提供了更多便利與選擇,也大大擴展了賭博產業的市場範圍。
**特點與魅力**
娛樂城和線上賭場的主要魅力在於它們能提供多樣化的娛樂選項和高度的可訪問性。無論是實體的娛樂城還是虛擬的線上賭場,它們都致力於創造一個充滿樂趣和刺激的環境,讓人們可以從日常生活的壓力中短暫逃脫。
此外,線上賭場通過提供豐富的遊戲選擇、吸引人的獎金方案以及便捷的支付系統,成功地吸引了全球範圍內的用戶。這些平台通常具有高度的互動性和社交性,使玩家不僅能享受遊戲本身,還能與來自世界各地的其他玩家交流。
**未來趨勢**
隨著技術的不斷進步和用戶需求的不斷演變,娛樂城和線上賭場的未來發展呈現出多元化的趨勢。一方面,虛
擬現實(VR)和擴增現實(AR)技術的應用,有望為線上賭場帶來更加沉浸式和互動式的遊戲體驗。另一方面,對於實體娛樂城而言,將更多地注重提供綜合性的休閒體驗,結合賭博、娛樂、休閒和旅遊等多個方面,以滿足不同客群的需求。
此外,隨著對負責任賭博的認識加深,未來娛樂城和線上賭場在提供娛樂的同時,也將更加注重促進健康的賭博行為和保護用戶的安全。
總之,娛樂城和線上賭場作為現代娛樂生活的一部分,隨著社會的發展和技術的進步,將繼續演化和創新,為人們提供更多的樂趣和便利。這一領域的未來發展無疑充滿了無限的可能性和機遇。
JustinAcige
Jan. 26, 2024, 3:47 p.m.
2024全新上線❰戰神賽特老虎機❱ - ATG賽特玩法說明介紹
❰戰神賽特老虎機❱是由ATG電子獨家開發的古埃及風格線上老虎機,在傳說中戰神賽特是「力量之神」與奈芙蒂斯女神結成連理,共同守護古埃及的奇幻秘寶,只有被選中的冒險者才能進入探險。
❰戰神賽特老虎機❱ – ATG賽特介紹
2024最新老虎機【戰神塞特】- ATG電子 X 富遊娛樂城
❰戰神賽特老虎機❱ – ATG電子
線上老虎機系統 : ATG電子
發行年分 : 2024年1月
最大倍數 : 51000倍
返還率 : 95.89%
支付方式 : 全盤倍數、消除掉落
最低投注金額 : 0.4元
最高投注金額 : 2000元
可否選台 : 是
可選台台數 : 350台
免費遊戲 : 選轉觸發+購買特色
❰戰神賽特老虎機❱ 賠率說明
戰神塞特老虎機賠率算法非常簡單,玩家們只需要不斷的轉動老虎機,成功消除物件即可贏分,得分賠率依賠率表計算。
當盤面上沒有物件可以消除時,倍數符號將會相加形成總倍數!該次旋轉的總贏分即為 : 贏分 X 總倍數。
積分方式如下 :
贏分=(單次押注額/20) X 物件賠率
EX : 單次押注額為1,盤面獲得12個戰神賽特倍數符號法老魔眼
贏分= (1/20) X 1000=50
以下為各個得分符號數量之獎金賠率 :
得分符號 獎金倍數 得分符號 獎金倍數
戰神賽特倍數符號聖甲蟲 6 2000
5 100
4 60 戰神賽特倍數符號黃寶石 12+ 200
10-11 30
8-9 20
戰神賽特倍數符號荷魯斯之眼 12+ 1000
10-11 500
8-9 200 戰神賽特倍數符號紅寶石 12+ 160
10-11 24
8-9 16
戰神賽特倍數符號眼鏡蛇 12+ 500
10-11 200
8-9 50 戰神賽特倍數符號紫鑽石 12+ 100
10-11 20
8-9 10
戰神賽特倍數符號神箭 12+ 300
10-11 100
8-9 40 戰神賽特倍數符號藍寶石 12+ 80
10-11 18
8-9 8
戰神賽特倍數符號屠鐮刀 12+ 240
10-11 40
8-9 30 戰神賽特倍數符號綠寶石 12+ 40
10-11 15
8-9 5
❰戰神賽特老虎機❱ 賠率說明(橘色數值為獲得數量、黑色數值為得分賠率)
ATG賽特 – 特色說明
ATG賽特 – 倍數符號獎金加乘
玩家們在看到盤面上出現倍數符號時,務必把握機會加速轉動ATG賽特老虎機,倍數符號會隨機出現2到500倍的隨機倍數。
當盤面無法在消除時,這些倍數總和會相加,乘上當時累積之獎金,即為最後得分總額。
倍數符號會出現在主遊戲和免費遊戲當中,玩家們千萬別錯過這個可以瞬間將得獎金額拉高的好機會!
ATG賽特 - 倍數符號獎金加乘
ATG賽特 – 倍數符號圖示
ATG賽特 – 進入神秘金字塔開啟免費遊戲
戰神賽特倍數符號聖甲蟲
❰戰神賽特老虎機❱ 免費遊戲符號
在古埃及神話中,聖甲蟲又稱為「死亡之蟲」,它被當成了天球及重生的象徵,守護古埃及的奇幻秘寶。
當玩家在盤面上,看見越來越多的聖甲蟲時,千萬不要膽怯,只需在牌面上斬殺4~6個ATG賽特免費遊戲符號,就可以進入15場免費遊戲!
在免費遊戲中若轉出3~6個聖甲蟲免費遊戲符號,可額外獲得5次免費遊戲,最高可達100次。
當玩家的累積贏分且盤面有倍數物件時,盤面上的所有倍數將會加入總倍數!
ATG賽特 – 選台模式贏在起跑線
為避免神聖的寶物被盜墓者奪走,富有智慧的法老王將金子塔內佈滿迷宮,有的設滿機關讓盜墓者寸步難行,有的暗藏機關可以直接前往存放神秘寶物的暗房。
ATG賽特老虎機設有350個機檯供玩家選擇,這是連魔龍老虎機、忍老虎機都給不出的機台數量,為的就是讓玩家,可以隨時進入神秘的古埃及的寶藏聖域,挖掘長眠已久的神祕寶藏。
【戰神塞特老虎機】選台模式
❰戰神賽特老虎機❱ 選台模式
ATG賽特 – 購買免費遊戲挖掘秘寶
玩家們可以使用當前投注額的100倍購買免費遊戲!進入免費遊戲再也不是虛幻。獎勵與一般遊戲相同,且購買後遊戲將自動開始,直到場次和獎金發放完畢為止。
有購買免費遊戲需求的玩家們,立即點擊「開始」,啟動神秘金字塔裡的古埃及祕寶吧!
【戰神塞特老虎機】購買特色
❰戰神賽特老虎機❱ 購買特色
戰神賽特試玩推薦
看完了❰戰神賽特老虎機❱介紹之後,玩家們是否也蓄勢待發要進入古埃及的世界,一探神奇秘寶探險之旅。
本次ATG賽特與線上娛樂城推薦第一名的富遊娛樂城合作,只需要加入會員,即可領取到168體驗金,免費試玩420轉!
JamesUnolf
Jan. 26, 2024, 3:47 p.m.
2024娛樂城No.1 – 富遊娛樂城介紹
2024 年 1 月 5 日
|
娛樂城, 現金版娛樂城
富遊娛樂城是無論老手、新手,都非常推薦的線上博奕,在2024娛樂城當中扮演著多年來最來勢洶洶的一匹黑馬,『人性化且精緻的介面,遊戲種類眾多,超級多的娛樂城優惠,擁有眾多與會員交流遊戲的群組』是一大特色。
富遊娛樂城擁有歐洲馬爾他(MGA)和菲律賓政府競猜委員會(PAGCOR)頒發的合法執照。
註冊於英屬維爾京群島,受國際行業協會認可的合法公司。
我們的中心思想就是能夠帶領玩家遠詐騙黑網,讓大家安心放心的暢玩線上博弈,娛樂城也受各大部落客、IG網紅、PTT論壇,推薦討論,富遊娛樂城沒有之一,絕對是線上賭場玩家的第一首選!
富遊娛樂城介面 / 2024娛樂城NO.1
富遊娛樂城簡介
品牌名稱 : 富遊RG
創立時間 : 2019年
存款速度 : 平均15秒
提款速度 : 平均5分
單筆提款金額 : 最低1000-100萬
遊戲對象 : 18歲以上男女老少皆可
合作廠商 : 22家遊戲平台商
支付平台 : 各大銀行、各大便利超商
支援配備 : 手機網頁、電腦網頁、IOS、安卓(Android)
富遊娛樂城遊戲品牌
真人百家 — 歐博真人、DG真人、亞博真人、SA真人、OG真人
體育投注 — SUPER體育、鑫寶體育、亞博體育
電競遊戲 — 泛亞電競
彩票遊戲 — 富遊彩票、WIN 539
電子遊戲 —ZG電子、BNG電子、BWIN電子、RSG電子、好路GR電子
棋牌遊戲 —ZG棋牌、亞博棋牌、好路棋牌、博亞棋牌
捕魚遊戲 —ZG捕魚、RSG捕魚、好路GR捕魚、亞博捕魚
富遊娛樂城優惠活動
每日任務簽到金666
富遊VIP全面啟動
復酬金活動10%優惠
日日返水
新會員好禮五選一
首存禮1000送1000
免費體驗金$168
富遊娛樂城APP
步驟1 : 開啟網頁版【富遊娛樂城官網】
步驟2 : 點選上方(下載app),會跳出下載與複製連結選項,點選後跳轉。
步驟3 : 跳轉後點選(安裝),並點選(允許)操作下載描述檔,跳出下載描述檔後點選關閉。
步驟4 : 到手機設置>一般>裝置管理>設定描述檔(富遊)安裝,即可完成安裝。
富遊娛樂城常見問題FAQ
富遊娛樂城詐騙?
黑網詐騙可細分兩種,小出大不出及純詐騙黑網,我們可從品牌知名度經營和網站架設畫面分辨來簡單分辨。
富遊娛樂城會出金嗎?
如上面提到,富遊是在做一個品牌,為的是能夠保證出金,和帶領玩家遠離黑網,而且還有DUKER娛樂城出金認證,所以各位能夠放心富遊娛樂城為正出金娛樂城。
富遊娛樂城出金延遲怎麼辦?
基本上只要是公司系統問提造成富遊娛樂城會員無法在30分鐘成功提款,富遊娛樂城會即刻派送補償金,表達誠摯的歉意。
富遊娛樂城結論
富遊娛樂城安心玩,評價4.5顆星。如果還想看其他娛樂城推薦的,可以來娛樂城推薦尋找喔。
BernardRigma
Jan. 26, 2024, 3:47 p.m.
2024娛樂城No.1 – 富遊娛樂城介紹
2024 年 1 月 5 日
|
娛樂城, 現金版娛樂城
富遊娛樂城是無論老手、新手,都非常推薦的線上博奕,在2024娛樂城當中扮演著多年來最來勢洶洶的一匹黑馬,『人性化且精緻的介面,遊戲種類眾多,超級多的娛樂城優惠,擁有眾多與會員交流遊戲的群組』是一大特色。
富遊娛樂城擁有歐洲馬爾他(MGA)和菲律賓政府競猜委員會(PAGCOR)頒發的合法執照。
註冊於英屬維爾京群島,受國際行業協會認可的合法公司。
我們的中心思想就是能夠帶領玩家遠詐騙黑網,讓大家安心放心的暢玩線上博弈,娛樂城也受各大部落客、IG網紅、PTT論壇,推薦討論,富遊娛樂城沒有之一,絕對是線上賭場玩家的第一首選!
富遊娛樂城介面 / 2024娛樂城NO.1
富遊娛樂城簡介
品牌名稱 : 富遊RG
創立時間 : 2019年
存款速度 : 平均15秒
提款速度 : 平均5分
單筆提款金額 : 最低1000-100萬
遊戲對象 : 18歲以上男女老少皆可
合作廠商 : 22家遊戲平台商
支付平台 : 各大銀行、各大便利超商
支援配備 : 手機網頁、電腦網頁、IOS、安卓(Android)
富遊娛樂城遊戲品牌
真人百家 — 歐博真人、DG真人、亞博真人、SA真人、OG真人
體育投注 — SUPER體育、鑫寶體育、亞博體育
電競遊戲 — 泛亞電競
彩票遊戲 — 富遊彩票、WIN 539
電子遊戲 —ZG電子、BNG電子、BWIN電子、RSG電子、好路GR電子
棋牌遊戲 —ZG棋牌、亞博棋牌、好路棋牌、博亞棋牌
捕魚遊戲 —ZG捕魚、RSG捕魚、好路GR捕魚、亞博捕魚
富遊娛樂城優惠活動
每日任務簽到金666
富遊VIP全面啟動
復酬金活動10%優惠
日日返水
新會員好禮五選一
首存禮1000送1000
免費體驗金$168
富遊娛樂城APP
步驟1 : 開啟網頁版【富遊娛樂城官網】
步驟2 : 點選上方(下載app),會跳出下載與複製連結選項,點選後跳轉。
步驟3 : 跳轉後點選(安裝),並點選(允許)操作下載描述檔,跳出下載描述檔後點選關閉。
步驟4 : 到手機設置>一般>裝置管理>設定描述檔(富遊)安裝,即可完成安裝。
富遊娛樂城常見問題FAQ
富遊娛樂城詐騙?
黑網詐騙可細分兩種,小出大不出及純詐騙黑網,我們可從品牌知名度經營和網站架設畫面分辨來簡單分辨。
富遊娛樂城會出金嗎?
如上面提到,富遊是在做一個品牌,為的是能夠保證出金,和帶領玩家遠離黑網,而且還有DUKER娛樂城出金認證,所以各位能夠放心富遊娛樂城為正出金娛樂城。
富遊娛樂城出金延遲怎麼辦?
基本上只要是公司系統問提造成富遊娛樂城會員無法在30分鐘成功提款,富遊娛樂城會即刻派送補償金,表達誠摯的歉意。
富遊娛樂城結論
富遊娛樂城安心玩,評價4.5顆星。如果還想看其他娛樂城推薦的,可以來娛樂城推薦尋找喔。
JustinAcige
Jan. 25, 2024, 6:35 a.m.
2024全新上線❰戰神賽特老虎機❱ - ATG賽特玩法說明介紹
❰戰神賽特老虎機❱是由ATG電子獨家開發的古埃及風格線上老虎機,在傳說中戰神賽特是「力量之神」與奈芙蒂斯女神結成連理,共同守護古埃及的奇幻秘寶,只有被選中的冒險者才能進入探險。
❰戰神賽特老虎機❱ – ATG賽特介紹
2024最新老虎機【戰神塞特】- ATG電子 X 富遊娛樂城
❰戰神賽特老虎機❱ – ATG電子
線上老虎機系統 : ATG電子
發行年分 : 2024年1月
最大倍數 : 51000倍
返還率 : 95.89%
支付方式 : 全盤倍數、消除掉落
最低投注金額 : 0.4元
最高投注金額 : 2000元
可否選台 : 是
可選台台數 : 350台
免費遊戲 : 選轉觸發+購買特色
❰戰神賽特老虎機❱ 賠率說明
戰神塞特老虎機賠率算法非常簡單,玩家們只需要不斷的轉動老虎機,成功消除物件即可贏分,得分賠率依賠率表計算。
當盤面上沒有物件可以消除時,倍數符號將會相加形成總倍數!該次旋轉的總贏分即為 : 贏分 X 總倍數。
積分方式如下 :
贏分=(單次押注額/20) X 物件賠率
EX : 單次押注額為1,盤面獲得12個戰神賽特倍數符號法老魔眼
贏分= (1/20) X 1000=50
以下為各個得分符號數量之獎金賠率 :
得分符號 獎金倍數 得分符號 獎金倍數
戰神賽特倍數符號聖甲蟲 6 2000
5 100
4 60 戰神賽特倍數符號黃寶石 12+ 200
10-11 30
8-9 20
戰神賽特倍數符號荷魯斯之眼 12+ 1000
10-11 500
8-9 200 戰神賽特倍數符號紅寶石 12+ 160
10-11 24
8-9 16
戰神賽特倍數符號眼鏡蛇 12+ 500
10-11 200
8-9 50 戰神賽特倍數符號紫鑽石 12+ 100
10-11 20
8-9 10
戰神賽特倍數符號神箭 12+ 300
10-11 100
8-9 40 戰神賽特倍數符號藍寶石 12+ 80
10-11 18
8-9 8
戰神賽特倍數符號屠鐮刀 12+ 240
10-11 40
8-9 30 戰神賽特倍數符號綠寶石 12+ 40
10-11 15
8-9 5
❰戰神賽特老虎機❱ 賠率說明(橘色數值為獲得數量、黑色數值為得分賠率)
ATG賽特 – 特色說明
ATG賽特 – 倍數符號獎金加乘
玩家們在看到盤面上出現倍數符號時,務必把握機會加速轉動ATG賽特老虎機,倍數符號會隨機出現2到500倍的隨機倍數。
當盤面無法在消除時,這些倍數總和會相加,乘上當時累積之獎金,即為最後得分總額。
倍數符號會出現在主遊戲和免費遊戲當中,玩家們千萬別錯過這個可以瞬間將得獎金額拉高的好機會!
ATG賽特 - 倍數符號獎金加乘
ATG賽特 – 倍數符號圖示
ATG賽特 – 進入神秘金字塔開啟免費遊戲
戰神賽特倍數符號聖甲蟲
❰戰神賽特老虎機❱ 免費遊戲符號
在古埃及神話中,聖甲蟲又稱為「死亡之蟲」,它被當成了天球及重生的象徵,守護古埃及的奇幻秘寶。
當玩家在盤面上,看見越來越多的聖甲蟲時,千萬不要膽怯,只需在牌面上斬殺4~6個ATG賽特免費遊戲符號,就可以進入15場免費遊戲!
在免費遊戲中若轉出3~6個聖甲蟲免費遊戲符號,可額外獲得5次免費遊戲,最高可達100次。
當玩家的累積贏分且盤面有倍數物件時,盤面上的所有倍數將會加入總倍數!
ATG賽特 – 選台模式贏在起跑線
為避免神聖的寶物被盜墓者奪走,富有智慧的法老王將金子塔內佈滿迷宮,有的設滿機關讓盜墓者寸步難行,有的暗藏機關可以直接前往存放神秘寶物的暗房。
ATG賽特老虎機設有350個機檯供玩家選擇,這是連魔龍老虎機、忍老虎機都給不出的機台數量,為的就是讓玩家,可以隨時進入神秘的古埃及的寶藏聖域,挖掘長眠已久的神祕寶藏。
【戰神塞特老虎機】選台模式
❰戰神賽特老虎機❱ 選台模式
ATG賽特 – 購買免費遊戲挖掘秘寶
玩家們可以使用當前投注額的100倍購買免費遊戲!進入免費遊戲再也不是虛幻。獎勵與一般遊戲相同,且購買後遊戲將自動開始,直到場次和獎金發放完畢為止。
有購買免費遊戲需求的玩家們,立即點擊「開始」,啟動神秘金字塔裡的古埃及祕寶吧!
【戰神塞特老虎機】購買特色
❰戰神賽特老虎機❱ 購買特色
戰神賽特試玩推薦
看完了❰戰神賽特老虎機❱介紹之後,玩家們是否也蓄勢待發要進入古埃及的世界,一探神奇秘寶探險之旅。
本次ATG賽特與線上娛樂城推薦第一名的富遊娛樂城合作,只需要加入會員,即可領取到168體驗金,免費試玩420轉!
BernardRigma
Jan. 24, 2024, 6:35 a.m.
Watches World: Elevating Luxury and Style with Exquisite Timepieces
Introduction:
Jewelry has always been a timeless expression of elegance, and nothing complements one's style better than a luxurious timepiece. At Watches World, we bring you an exclusive collection of coveted luxury watches that not only tell time but also serve as a testament to your refined taste. Explore our curated selection featuring iconic brands like Rolex, Hublot, Omega, Cartier, and more, as we redefine the art of accessorizing.
A Dazzling Array of Luxury Watches:
Watches World offers an unparalleled range of exquisite timepieces from renowned brands, ensuring that you find the perfect accessory to elevate your style. Whether you're drawn to the classic sophistication of Rolex, the avant-garde designs of Hublot, or the precision engineering of Patek Philippe, our collection caters to diverse preferences.
Customer Testimonials:
Our commitment to providing an exceptional customer experience is reflected in the reviews from our satisfied clientele. O.M. commends our excellent communication and flawless packaging, while Richard Houtman appreciates the helpfulness and courtesy of our team. These testimonials highlight our dedication to transparency, communication, and customer satisfaction.
New Arrivals:
Stay ahead of the curve with our latest additions, including the Tudor Black Bay Ceramic 41mm, Richard Mille RM35-01 Rafael Nadal NTPT Carbon Limited Edition, and the Rolex Oyster Perpetual Datejust 41mm series. These new arrivals showcase cutting-edge designs and impeccable craftsmanship, ensuring you stay on the forefront of luxury watch fashion.
Best Sellers:
Discover our best-selling watches, such as the Bulgari Serpenti Tubogas 35mm and the Cartier Panthere Medium Model. These timeless pieces combine elegance with precision, making them a staple in any sophisticated wardrobe.
Expert's Selection:
Our experts have carefully curated a selection of watches, including the Cartier Panthere Small Model, Omega Speedmaster Moonwatch 44.25 mm, and Rolex Oyster Perpetual Cosmograph Daytona 40mm. These choices exemplify the epitome of watchmaking excellence and style.
Secured and Tracked Delivery:
At Watches World, we prioritize the safety of your purchase. Our secured and tracked delivery ensures that your exquisite timepiece reaches you in perfect condition, giving you peace of mind with every order.
Passionate Experts at Your Service:
Our team of passionate watch experts is dedicated to providing personalized service. From assisting you in choosing the perfect timepiece to addressing any inquiries, we are here to make your watch-buying experience seamless and enjoyable.
Global Presence:
With a presence in key cities around the world, including Dubai, Geneva, Hong Kong, London, Miami, Paris, Prague, Dublin, Singapore, and Sao Paulo, Watches World brings luxury timepieces to enthusiasts globally.
Conclusion:
Watches World goes beyond being an online platform for luxury watches; it is a destination where expertise, trust, and satisfaction converge. Explore our collection, and let our timeless timepieces become an integral part of your style narrative. Join us in redefining luxury, one exquisite watch at a time.
BernardRigma
Jan. 23, 2024, 6:35 a.m.
Watches World: Elevating Luxury and Style with Exquisite Timepieces
Introduction:
Jewelry has always been a timeless expression of elegance, and nothing complements one's style better than a luxurious timepiece. At Watches World, we bring you an exclusive collection of coveted luxury watches that not only tell time but also serve as a testament to your refined taste. Explore our curated selection featuring iconic brands like Rolex, Hublot, Omega, Cartier, and more, as we redefine the art of accessorizing.
A Dazzling Array of Luxury Watches:
Watches World offers an unparalleled range of exquisite timepieces from renowned brands, ensuring that you find the perfect accessory to elevate your style. Whether you're drawn to the classic sophistication of Rolex, the avant-garde designs of Hublot, or the precision engineering of Patek Philippe, our collection caters to diverse preferences.
Customer Testimonials:
Our commitment to providing an exceptional customer experience is reflected in the reviews from our satisfied clientele. O.M. commends our excellent communication and flawless packaging, while Richard Houtman appreciates the helpfulness and courtesy of our team. These testimonials highlight our dedication to transparency, communication, and customer satisfaction.
New Arrivals:
Stay ahead of the curve with our latest additions, including the Tudor Black Bay Ceramic 41mm, Richard Mille RM35-01 Rafael Nadal NTPT Carbon Limited Edition, and the Rolex Oyster Perpetual Datejust 41mm series. These new arrivals showcase cutting-edge designs and impeccable craftsmanship, ensuring you stay on the forefront of luxury watch fashion.
Best Sellers:
Discover our best-selling watches, such as the Bulgari Serpenti Tubogas 35mm and the Cartier Panthere Medium Model. These timeless pieces combine elegance with precision, making them a staple in any sophisticated wardrobe.
Expert's Selection:
Our experts have carefully curated a selection of watches, including the Cartier Panthere Small Model, Omega Speedmaster Moonwatch 44.25 mm, and Rolex Oyster Perpetual Cosmograph Daytona 40mm. These choices exemplify the epitome of watchmaking excellence and style.
Secured and Tracked Delivery:
At Watches World, we prioritize the safety of your purchase. Our secured and tracked delivery ensures that your exquisite timepiece reaches you in perfect condition, giving you peace of mind with every order.
Passionate Experts at Your Service:
Our team of passionate watch experts is dedicated to providing personalized service. From assisting you in choosing the perfect timepiece to addressing any inquiries, we are here to make your watch-buying experience seamless and enjoyable.
Global Presence:
With a presence in key cities around the world, including Dubai, Geneva, Hong Kong, London, Miami, Paris, Prague, Dublin, Singapore, and Sao Paulo, Watches World brings luxury timepieces to enthusiasts globally.
Conclusion:
Watches World goes beyond being an online platform for luxury watches; it is a destination where expertise, trust, and satisfaction converge. Explore our collection, and let our timeless timepieces become an integral part of your style narrative. Join us in redefining luxury, one exquisite watch at a time.
BernardRigma
Jan. 22, 2024, 6:35 a.m.
Watches World: Elevating Luxury and Style with Exquisite Timepieces
Introduction:
Jewelry has always been a timeless expression of elegance, and nothing complements one's style better than a luxurious timepiece. At Watches World, we bring you an exclusive collection of coveted luxury watches that not only tell time but also serve as a testament to your refined taste. Explore our curated selection featuring iconic brands like Rolex, Hublot, Omega, Cartier, and more, as we redefine the art of accessorizing.
A Dazzling Array of Luxury Watches:
Watches World offers an unparalleled range of exquisite timepieces from renowned brands, ensuring that you find the perfect accessory to elevate your style. Whether you're drawn to the classic sophistication of Rolex, the avant-garde designs of Hublot, or the precision engineering of Patek Philippe, our collection caters to diverse preferences.
Customer Testimonials:
Our commitment to providing an exceptional customer experience is reflected in the reviews from our satisfied clientele. O.M. commends our excellent communication and flawless packaging, while Richard Houtman appreciates the helpfulness and courtesy of our team. These testimonials highlight our dedication to transparency, communication, and customer satisfaction.
New Arrivals:
Stay ahead of the curve with our latest additions, including the Tudor Black Bay Ceramic 41mm, Richard Mille RM35-01 Rafael Nadal NTPT Carbon Limited Edition, and the Rolex Oyster Perpetual Datejust 41mm series. These new arrivals showcase cutting-edge designs and impeccable craftsmanship, ensuring you stay on the forefront of luxury watch fashion.
Best Sellers:
Discover our best-selling watches, such as the Bulgari Serpenti Tubogas 35mm and the Cartier Panthere Medium Model. These timeless pieces combine elegance with precision, making them a staple in any sophisticated wardrobe.
Expert's Selection:
Our experts have carefully curated a selection of watches, including the Cartier Panthere Small Model, Omega Speedmaster Moonwatch 44.25 mm, and Rolex Oyster Perpetual Cosmograph Daytona 40mm. These choices exemplify the epitome of watchmaking excellence and style.
Secured and Tracked Delivery:
At Watches World, we prioritize the safety of your purchase. Our secured and tracked delivery ensures that your exquisite timepiece reaches you in perfect condition, giving you peace of mind with every order.
Passionate Experts at Your Service:
Our team of passionate watch experts is dedicated to providing personalized service. From assisting you in choosing the perfect timepiece to addressing any inquiries, we are here to make your watch-buying experience seamless and enjoyable.
Global Presence:
With a presence in key cities around the world, including Dubai, Geneva, Hong Kong, London, Miami, Paris, Prague, Dublin, Singapore, and Sao Paulo, Watches World brings luxury timepieces to enthusiasts globally.
Conclusion:
Watches World goes beyond being an online platform for luxury watches; it is a destination where expertise, trust, and satisfaction converge. Explore our collection, and let our timeless timepieces become an integral part of your style narrative. Join us in redefining luxury, one exquisite watch at a time.
EdwardApota
Jan. 19, 2024, 6:35 a.m.
2024娛樂城的創新趨勢
隨著2024年的到來,娛樂城業界正經歷著一場革命性的變遷。這一年,娛樂城不僅僅是賭博和娛樂的代名詞,更成為了科技創新和用戶體驗的集大成者。
首先,2024年的娛樂城極大地融合了最新的技術。增強現實(AR)和虛擬現實(VR)技術的引入,為玩家提供了沉浸式的賭博體驗。這種全新的遊戲方式不僅帶來視覺上的震撼,還為玩家創造了一種置身於真實賭場的感覺,而實際上他們可能只是坐在家中的沙發上。
其次,人工智能(AI)在娛樂城中的應用也達到了新高度。AI技術不僅用於增強遊戲的公平性和透明度,還在個性化玩家體驗方面發揮著重要作用。從個性化遊戲推薦到智能客服,AI的應用使得娛樂城更能滿足玩家的個別需求。
此外,線上娛樂城的安全性和隱私保護也獲得了顯著加強。隨著技術的進步,更加先進的加密技術和安全措施被用來保護玩家的資料和交易,從而確保一個安全可靠的遊戲環境。
2024年的娛樂城還強調負責任的賭博。許多平台採用了各種工具和資源來幫助玩家控制他們的賭博行為,如設置賭注限制、自我排除措施等,體現了對可持續賭博的承諾。
總之,2024年的娛樂城呈現出一個高度融合了技術、安全和負責任賭博的行業新面貌,為玩家提供了前所未有的娛樂體驗。隨著這些趨勢的持續發展,我們可以預見,娛樂城將不斷地創新和進步,為玩家帶來更多精彩和安全的娛樂選擇。
JamesUnolf
Jan. 18, 2024, 6:35 a.m.
2024娛樂城的創新趨勢
隨著2024年的到來,娛樂城業界正經歷著一場革命性的變遷。這一年,娛樂城不僅僅是賭博和娛樂的代名詞,更成為了科技創新和用戶體驗的集大成者。
首先,2024年的娛樂城極大地融合了最新的技術。增強現實(AR)和虛擬現實(VR)技術的引入,為玩家提供了沉浸式的賭博體驗。這種全新的遊戲方式不僅帶來視覺上的震撼,還為玩家創造了一種置身於真實賭場的感覺,而實際上他們可能只是坐在家中的沙發上。
其次,人工智能(AI)在娛樂城中的應用也達到了新高度。AI技術不僅用於增強遊戲的公平性和透明度,還在個性化玩家體驗方面發揮著重要作用。從個性化遊戲推薦到智能客服,AI的應用使得娛樂城更能滿足玩家的個別需求。
此外,線上娛樂城的安全性和隱私保護也獲得了顯著加強。隨著技術的進步,更加先進的加密技術和安全措施被用來保護玩家的資料和交易,從而確保一個安全可靠的遊戲環境。
2024年的娛樂城還強調負責任的賭博。許多平台採用了各種工具和資源來幫助玩家控制他們的賭博行為,如設置賭注限制、自我排除措施等,體現了對可持續賭博的承諾。
總之,2024年的娛樂城呈現出一個高度融合了技術、安全和負責任賭博的行業新面貌,為玩家提供了前所未有的娛樂體驗。隨著這些趨勢的持續發展,我們可以預見,娛樂城將不斷地創新和進步,為玩家帶來更多精彩和安全的娛樂選擇。
BernardRigma
Jan. 6, 2024, 6:35 a.m.
民意調查是什麼?民調什麼意思?
民意調查又稱為輿論調查或民意測驗,簡稱民調。一般而言,民調是一種為了解公眾對某些政治、社會問題與政策的意見和態度,由專業民調公司或媒體進行的調查方法。
目的在於通過網路、電話、或書面等媒介,對大量樣本的問卷調查抽樣,利用統計學的抽樣理論來推斷較為客觀,且能較為精確地推論社會輿論或民意動向的一種方法。
以下是民意調查的一些基本特點和重要性:
抽樣:由於不可能向每一個人詢問意見,所以調查者會選擇一個代表性的樣本進行調查。這樣本的大小和抽樣方法都會影響調查的準確性和可靠性。
問卷設計:為了確保獲得可靠的結果,問卷必須經過精心設計,問題要清晰、不帶偏見,且易於理解。
數據分析:收集到的數據將被分析以得出結論。這可能包括計算百分比、平均值、標準差等,以及更複雜的統計分析。
多種用途:民意調查可以用於各種目的,包括政策制定、選舉預測、市場研究、社會科學研究等。
限制:雖然民意調查是一個有價值的工具,但它也有其限制。例如,樣本可能不完全代表目標人群,或者問卷的設計可能導致偏見。
影響決策:民意調查的結果常常被政府、企業和其他組織用來影響其決策。
透明度和誠實:為了維護調查的可信度,調查組織應該提供其調查方法、樣本大小、抽樣方法和可能的誤差範圍等詳細資訊。
民調是怎麼調查的?
民意調查(輿論調查)的意義是指為瞭解大多數民眾的看法、意見、利益與需求,以科學、系統與公正的資料,蒐集可以代表全部群眾(母體)的部分群眾(抽樣),設計問卷題目後,以人工或電腦詢問部分民眾對特定議題的看法與評價,利用抽樣出來部分民眾的意見與看法,來推論目前全部民眾的意見與看法,藉以衡量社會與政治的狀態。
以下是進行民調調查的基本步驟:
定義目標和目的:首先,調查者需要明確調查的目的。是要了解公眾對某個政策的看法?還是要評估某個政治候選人的支持率?
設計問卷:根據調查目的,研究者會設計一份問卷。問卷應該包含清晰、不帶偏見的問題,並避免導向性的語言。
選擇樣本:因為通常不可能調查所有人,所以會選擇一部分人作為代表。這部分人被稱為“樣本”。最理想的情況是使用隨機抽樣,以確保每個人都有被選中的機會。
收集數據:有多種方法可以收集數據,如面對面訪問、電話訪問、郵件調查或在線調查。
數據分析:一旦數據被收集,研究者會使用統計工具和技術進行分析,得出結論或洞見。
報告結果:分析完數據後,研究者會編寫報告或發布結果。報告通常會提供調查方法、樣本大小、誤差範圍和主要發現。
解釋誤差範圍:多數民調報告都會提供誤差範圍,例如“±3%”。這表示實際的結果有可能在報告結果的3%範圍內上下浮動。
民調調查的質量和可信度很大程度上取決於其設計和實施的方法。若是由專業和無偏見的組織進行,且使用科學的方法,那麼民調結果往往較為可靠。但即使是最高質量的民調也會有一定的誤差,因此解讀時應保持批判性思考。
為什麼要做民調?
民調提供了一種系統性的方式來了解大眾的意見、態度和信念。進行民調的原因多種多樣,以下是一些主要的動機:
政策制定和評估:政府和政策制定者進行民調,以了解公眾對某一議題或政策的看法。這有助於制定或調整政策,以反映大眾的需求和意見。
選舉和政治活動:政黨和候選人通常使用民調來評估自己在選舉中的地位,了解哪些議題對選民最重要,以及如何調整策略以吸引更多支持。
市場研究:企業和組織進行民調以了解消費者對產品、服務或品牌的態度,從而制定或調整市場策略。
社會科學研究:學者和研究者使用民調來了解人們的社會、文化和心理特征,以及其與行為的關係。
公眾與媒體的期望:民調提供了一種方式,使公眾、政府和企業得以了解社會的整體趨勢和態度。媒體也經常報導民調結果,提供公眾對當前議題的見解。
提供反饋和評估:無論是企業還是政府,都可以透過民調了解其表現、服務或政策的效果,並根據反饋進行改進。
預測和趨勢分析:民調可以幫助預測某些趨勢或行為的未來發展,如選舉結果、市場需求等。
教育和提高公眾意識:通過進行和公布民調,可以促使公眾對某一議題或問題有更深入的了解和討論。
民調可信嗎?
民意調查的結果數據隨處可見,尤其是政治性民調結果幾乎可說是天天在新聞上放送,對總統的滿意度下降了多少百分比,然而大家又信多少?
在景美市場的訪問中,我們了解到民眾對民調有一些普遍的觀點。大多數受訪者表示,他們對民調的可信度存有疑慮,主要原因是他們擔心政府可能會在調查中進行操控,以符合特定政治目標。
受訪者還提到,民意調查的結果通常不會對他們的投票意願產生影響。換句話說,他們的選擇通常受到更多因素的影響,例如候選人的政策立場和政府做事的認真與否,而不是單純依賴民調結果。
從訪問中我們可以得出的結論是,大多數民眾對民調持謹慎態度,並認為它們對他們的投票決策影響有限。
JamesUnolf
Jan. 6, 2024, 6:35 a.m.
民意調查是什麼?民調什麼意思?
民意調查又稱為輿論調查或民意測驗,簡稱民調。一般而言,民調是一種為了解公眾對某些政治、社會問題與政策的意見和態度,由專業民調公司或媒體進行的調查方法。
目的在於通過網路、電話、或書面等媒介,對大量樣本的問卷調查抽樣,利用統計學的抽樣理論來推斷較為客觀,且能較為精確地推論社會輿論或民意動向的一種方法。
以下是民意調查的一些基本特點和重要性:
抽樣:由於不可能向每一個人詢問意見,所以調查者會選擇一個代表性的樣本進行調查。這樣本的大小和抽樣方法都會影響調查的準確性和可靠性。
問卷設計:為了確保獲得可靠的結果,問卷必須經過精心設計,問題要清晰、不帶偏見,且易於理解。
數據分析:收集到的數據將被分析以得出結論。這可能包括計算百分比、平均值、標準差等,以及更複雜的統計分析。
多種用途:民意調查可以用於各種目的,包括政策制定、選舉預測、市場研究、社會科學研究等。
限制:雖然民意調查是一個有價值的工具,但它也有其限制。例如,樣本可能不完全代表目標人群,或者問卷的設計可能導致偏見。
影響決策:民意調查的結果常常被政府、企業和其他組織用來影響其決策。
透明度和誠實:為了維護調查的可信度,調查組織應該提供其調查方法、樣本大小、抽樣方法和可能的誤差範圍等詳細資訊。
民調是怎麼調查的?
民意調查(輿論調查)的意義是指為瞭解大多數民眾的看法、意見、利益與需求,以科學、系統與公正的資料,蒐集可以代表全部群眾(母體)的部分群眾(抽樣),設計問卷題目後,以人工或電腦詢問部分民眾對特定議題的看法與評價,利用抽樣出來部分民眾的意見與看法,來推論目前全部民眾的意見與看法,藉以衡量社會與政治的狀態。
以下是進行民調調查的基本步驟:
定義目標和目的:首先,調查者需要明確調查的目的。是要了解公眾對某個政策的看法?還是要評估某個政治候選人的支持率?
設計問卷:根據調查目的,研究者會設計一份問卷。問卷應該包含清晰、不帶偏見的問題,並避免導向性的語言。
選擇樣本:因為通常不可能調查所有人,所以會選擇一部分人作為代表。這部分人被稱為“樣本”。最理想的情況是使用隨機抽樣,以確保每個人都有被選中的機會。
收集數據:有多種方法可以收集數據,如面對面訪問、電話訪問、郵件調查或在線調查。
數據分析:一旦數據被收集,研究者會使用統計工具和技術進行分析,得出結論或洞見。
報告結果:分析完數據後,研究者會編寫報告或發布結果。報告通常會提供調查方法、樣本大小、誤差範圍和主要發現。
解釋誤差範圍:多數民調報告都會提供誤差範圍,例如“±3%”。這表示實際的結果有可能在報告結果的3%範圍內上下浮動。
民調調查的質量和可信度很大程度上取決於其設計和實施的方法。若是由專業和無偏見的組織進行,且使用科學的方法,那麼民調結果往往較為可靠。但即使是最高質量的民調也會有一定的誤差,因此解讀時應保持批判性思考。
為什麼要做民調?
民調提供了一種系統性的方式來了解大眾的意見、態度和信念。進行民調的原因多種多樣,以下是一些主要的動機:
政策制定和評估:政府和政策制定者進行民調,以了解公眾對某一議題或政策的看法。這有助於制定或調整政策,以反映大眾的需求和意見。
選舉和政治活動:政黨和候選人通常使用民調來評估自己在選舉中的地位,了解哪些議題對選民最重要,以及如何調整策略以吸引更多支持。
市場研究:企業和組織進行民調以了解消費者對產品、服務或品牌的態度,從而制定或調整市場策略。
社會科學研究:學者和研究者使用民調來了解人們的社會、文化和心理特征,以及其與行為的關係。
公眾與媒體的期望:民調提供了一種方式,使公眾、政府和企業得以了解社會的整體趨勢和態度。媒體也經常報導民調結果,提供公眾對當前議題的見解。
提供反饋和評估:無論是企業還是政府,都可以透過民調了解其表現、服務或政策的效果,並根據反饋進行改進。
預測和趨勢分析:民調可以幫助預測某些趨勢或行為的未來發展,如選舉結果、市場需求等。
教育和提高公眾意識:通過進行和公布民調,可以促使公眾對某一議題或問題有更深入的了解和討論。
民調可信嗎?
民意調查的結果數據隨處可見,尤其是政治性民調結果幾乎可說是天天在新聞上放送,對總統的滿意度下降了多少百分比,然而大家又信多少?
在景美市場的訪問中,我們了解到民眾對民調有一些普遍的觀點。大多數受訪者表示,他們對民調的可信度存有疑慮,主要原因是他們擔心政府可能會在調查中進行操控,以符合特定政治目標。
受訪者還提到,民意調查的結果通常不會對他們的投票意願產生影響。換句話說,他們的選擇通常受到更多因素的影響,例如候選人的政策立場和政府做事的認真與否,而不是單純依賴民調結果。
從訪問中我們可以得出的結論是,大多數民眾對民調持謹慎態度,並認為它們對他們的投票決策影響有限。
JamesUnolf
Jan. 6, 2024, 3:57 a.m.
民意調查是什麼?民調什麼意思?
民意調查又稱為輿論調查或民意測驗,簡稱民調。一般而言,民調是一種為了解公眾對某些政治、社會問題與政策的意見和態度,由專業民調公司或媒體進行的調查方法。
目的在於通過網路、電話、或書面等媒介,對大量樣本的問卷調查抽樣,利用統計學的抽樣理論來推斷較為客觀,且能較為精確地推論社會輿論或民意動向的一種方法。
以下是民意調查的一些基本特點和重要性:
抽樣:由於不可能向每一個人詢問意見,所以調查者會選擇一個代表性的樣本進行調查。這樣本的大小和抽樣方法都會影響調查的準確性和可靠性。
問卷設計:為了確保獲得可靠的結果,問卷必須經過精心設計,問題要清晰、不帶偏見,且易於理解。
數據分析:收集到的數據將被分析以得出結論。這可能包括計算百分比、平均值、標準差等,以及更複雜的統計分析。
多種用途:民意調查可以用於各種目的,包括政策制定、選舉預測、市場研究、社會科學研究等。
限制:雖然民意調查是一個有價值的工具,但它也有其限制。例如,樣本可能不完全代表目標人群,或者問卷的設計可能導致偏見。
影響決策:民意調查的結果常常被政府、企業和其他組織用來影響其決策。
透明度和誠實:為了維護調查的可信度,調查組織應該提供其調查方法、樣本大小、抽樣方法和可能的誤差範圍等詳細資訊。
民調是怎麼調查的?
民意調查(輿論調查)的意義是指為瞭解大多數民眾的看法、意見、利益與需求,以科學、系統與公正的資料,蒐集可以代表全部群眾(母體)的部分群眾(抽樣),設計問卷題目後,以人工或電腦詢問部分民眾對特定議題的看法與評價,利用抽樣出來部分民眾的意見與看法,來推論目前全部民眾的意見與看法,藉以衡量社會與政治的狀態。
以下是進行民調調查的基本步驟:
定義目標和目的:首先,調查者需要明確調查的目的。是要了解公眾對某個政策的看法?還是要評估某個政治候選人的支持率?
設計問卷:根據調查目的,研究者會設計一份問卷。問卷應該包含清晰、不帶偏見的問題,並避免導向性的語言。
選擇樣本:因為通常不可能調查所有人,所以會選擇一部分人作為代表。這部分人被稱為“樣本”。最理想的情況是使用隨機抽樣,以確保每個人都有被選中的機會。
收集數據:有多種方法可以收集數據,如面對面訪問、電話訪問、郵件調查或在線調查。
數據分析:一旦數據被收集,研究者會使用統計工具和技術進行分析,得出結論或洞見。
報告結果:分析完數據後,研究者會編寫報告或發布結果。報告通常會提供調查方法、樣本大小、誤差範圍和主要發現。
解釋誤差範圍:多數民調報告都會提供誤差範圍,例如“±3%”。這表示實際的結果有可能在報告結果的3%範圍內上下浮動。
民調調查的質量和可信度很大程度上取決於其設計和實施的方法。若是由專業和無偏見的組織進行,且使用科學的方法,那麼民調結果往往較為可靠。但即使是最高質量的民調也會有一定的誤差,因此解讀時應保持批判性思考。
為什麼要做民調?
民調提供了一種系統性的方式來了解大眾的意見、態度和信念。進行民調的原因多種多樣,以下是一些主要的動機:
政策制定和評估:政府和政策制定者進行民調,以了解公眾對某一議題或政策的看法。這有助於制定或調整政策,以反映大眾的需求和意見。
選舉和政治活動:政黨和候選人通常使用民調來評估自己在選舉中的地位,了解哪些議題對選民最重要,以及如何調整策略以吸引更多支持。
市場研究:企業和組織進行民調以了解消費者對產品、服務或品牌的態度,從而制定或調整市場策略。
社會科學研究:學者和研究者使用民調來了解人們的社會、文化和心理特征,以及其與行為的關係。
公眾與媒體的期望:民調提供了一種方式,使公眾、政府和企業得以了解社會的整體趨勢和態度。媒體也經常報導民調結果,提供公眾對當前議題的見解。
提供反饋和評估:無論是企業還是政府,都可以透過民調了解其表現、服務或政策的效果,並根據反饋進行改進。
預測和趨勢分析:民調可以幫助預測某些趨勢或行為的未來發展,如選舉結果、市場需求等。
教育和提高公眾意識:通過進行和公布民調,可以促使公眾對某一議題或問題有更深入的了解和討論。
民調可信嗎?
民意調查的結果數據隨處可見,尤其是政治性民調結果幾乎可說是天天在新聞上放送,對總統的滿意度下降了多少百分比,然而大家又信多少?
在景美市場的訪問中,我們了解到民眾對民調有一些普遍的觀點。大多數受訪者表示,他們對民調的可信度存有疑慮,主要原因是他們擔心政府可能會在調查中進行操控,以符合特定政治目標。
受訪者還提到,民意調查的結果通常不會對他們的投票意願產生影響。換句話說,他們的選擇通常受到更多因素的影響,例如候選人的政策立場和政府做事的認真與否,而不是單純依賴民調結果。
從訪問中我們可以得出的結論是,大多數民眾對民調持謹慎態度,並認為它們對他們的投票決策影響有限。
JamesUnolf
Jan. 5, 2024, 6:35 a.m.
民意調查是什麼?民調什麼意思?
民意調查又稱為輿論調查或民意測驗,簡稱民調。一般而言,民調是一種為了解公眾對某些政治、社會問題與政策的意見和態度,由專業民調公司或媒體進行的調查方法。
目的在於通過網路、電話、或書面等媒介,對大量樣本的問卷調查抽樣,利用統計學的抽樣理論來推斷較為客觀,且能較為精確地推論社會輿論或民意動向的一種方法。
以下是民意調查的一些基本特點和重要性:
抽樣:由於不可能向每一個人詢問意見,所以調查者會選擇一個代表性的樣本進行調查。這樣本的大小和抽樣方法都會影響調查的準確性和可靠性。
問卷設計:為了確保獲得可靠的結果,問卷必須經過精心設計,問題要清晰、不帶偏見,且易於理解。
數據分析:收集到的數據將被分析以得出結論。這可能包括計算百分比、平均值、標準差等,以及更複雜的統計分析。
多種用途:民意調查可以用於各種目的,包括政策制定、選舉預測、市場研究、社會科學研究等。
限制:雖然民意調查是一個有價值的工具,但它也有其限制。例如,樣本可能不完全代表目標人群,或者問卷的設計可能導致偏見。
影響決策:民意調查的結果常常被政府、企業和其他組織用來影響其決策。
透明度和誠實:為了維護調查的可信度,調查組織應該提供其調查方法、樣本大小、抽樣方法和可能的誤差範圍等詳細資訊。
民調是怎麼調查的?
民意調查(輿論調查)的意義是指為瞭解大多數民眾的看法、意見、利益與需求,以科學、系統與公正的資料,蒐集可以代表全部群眾(母體)的部分群眾(抽樣),設計問卷題目後,以人工或電腦詢問部分民眾對特定議題的看法與評價,利用抽樣出來部分民眾的意見與看法,來推論目前全部民眾的意見與看法,藉以衡量社會與政治的狀態。
以下是進行民調調查的基本步驟:
定義目標和目的:首先,調查者需要明確調查的目的。是要了解公眾對某個政策的看法?還是要評估某個政治候選人的支持率?
設計問卷:根據調查目的,研究者會設計一份問卷。問卷應該包含清晰、不帶偏見的問題,並避免導向性的語言。
選擇樣本:因為通常不可能調查所有人,所以會選擇一部分人作為代表。這部分人被稱為“樣本”。最理想的情況是使用隨機抽樣,以確保每個人都有被選中的機會。
收集數據:有多種方法可以收集數據,如面對面訪問、電話訪問、郵件調查或在線調查。
數據分析:一旦數據被收集,研究者會使用統計工具和技術進行分析,得出結論或洞見。
報告結果:分析完數據後,研究者會編寫報告或發布結果。報告通常會提供調查方法、樣本大小、誤差範圍和主要發現。
解釋誤差範圍:多數民調報告都會提供誤差範圍,例如“±3%”。這表示實際的結果有可能在報告結果的3%範圍內上下浮動。
民調調查的質量和可信度很大程度上取決於其設計和實施的方法。若是由專業和無偏見的組織進行,且使用科學的方法,那麼民調結果往往較為可靠。但即使是最高質量的民調也會有一定的誤差,因此解讀時應保持批判性思考。
為什麼要做民調?
民調提供了一種系統性的方式來了解大眾的意見、態度和信念。進行民調的原因多種多樣,以下是一些主要的動機:
政策制定和評估:政府和政策制定者進行民調,以了解公眾對某一議題或政策的看法。這有助於制定或調整政策,以反映大眾的需求和意見。
選舉和政治活動:政黨和候選人通常使用民調來評估自己在選舉中的地位,了解哪些議題對選民最重要,以及如何調整策略以吸引更多支持。
市場研究:企業和組織進行民調以了解消費者對產品、服務或品牌的態度,從而制定或調整市場策略。
社會科學研究:學者和研究者使用民調來了解人們的社會、文化和心理特征,以及其與行為的關係。
公眾與媒體的期望:民調提供了一種方式,使公眾、政府和企業得以了解社會的整體趨勢和態度。媒體也經常報導民調結果,提供公眾對當前議題的見解。
提供反饋和評估:無論是企業還是政府,都可以透過民調了解其表現、服務或政策的效果,並根據反饋進行改進。
預測和趨勢分析:民調可以幫助預測某些趨勢或行為的未來發展,如選舉結果、市場需求等。
教育和提高公眾意識:通過進行和公布民調,可以促使公眾對某一議題或問題有更深入的了解和討論。
民調可信嗎?
民意調查的結果數據隨處可見,尤其是政治性民調結果幾乎可說是天天在新聞上放送,對總統的滿意度下降了多少百分比,然而大家又信多少?
在景美市場的訪問中,我們了解到民眾對民調有一些普遍的觀點。大多數受訪者表示,他們對民調的可信度存有疑慮,主要原因是他們擔心政府可能會在調查中進行操控,以符合特定政治目標。
受訪者還提到,民意調查的結果通常不會對他們的投票意願產生影響。換句話說,他們的選擇通常受到更多因素的影響,例如候選人的政策立場和政府做事的認真與否,而不是單純依賴民調結果。
從訪問中我們可以得出的結論是,大多數民眾對民調持謹慎態度,並認為它們對他們的投票決策影響有限。
BernardRigma
Jan. 5, 2024, 6:35 a.m.
民意調查是什麼?民調什麼意思?
民意調查又稱為輿論調查或民意測驗,簡稱民調。一般而言,民調是一種為了解公眾對某些政治、社會問題與政策的意見和態度,由專業民調公司或媒體進行的調查方法。
目的在於通過網路、電話、或書面等媒介,對大量樣本的問卷調查抽樣,利用統計學的抽樣理論來推斷較為客觀,且能較為精確地推論社會輿論或民意動向的一種方法。
以下是民意調查的一些基本特點和重要性:
抽樣:由於不可能向每一個人詢問意見,所以調查者會選擇一個代表性的樣本進行調查。這樣本的大小和抽樣方法都會影響調查的準確性和可靠性。
問卷設計:為了確保獲得可靠的結果,問卷必須經過精心設計,問題要清晰、不帶偏見,且易於理解。
數據分析:收集到的數據將被分析以得出結論。這可能包括計算百分比、平均值、標準差等,以及更複雜的統計分析。
多種用途:民意調查可以用於各種目的,包括政策制定、選舉預測、市場研究、社會科學研究等。
限制:雖然民意調查是一個有價值的工具,但它也有其限制。例如,樣本可能不完全代表目標人群,或者問卷的設計可能導致偏見。
影響決策:民意調查的結果常常被政府、企業和其他組織用來影響其決策。
透明度和誠實:為了維護調查的可信度,調查組織應該提供其調查方法、樣本大小、抽樣方法和可能的誤差範圍等詳細資訊。
民調是怎麼調查的?
民意調查(輿論調查)的意義是指為瞭解大多數民眾的看法、意見、利益與需求,以科學、系統與公正的資料,蒐集可以代表全部群眾(母體)的部分群眾(抽樣),設計問卷題目後,以人工或電腦詢問部分民眾對特定議題的看法與評價,利用抽樣出來部分民眾的意見與看法,來推論目前全部民眾的意見與看法,藉以衡量社會與政治的狀態。
以下是進行民調調查的基本步驟:
定義目標和目的:首先,調查者需要明確調查的目的。是要了解公眾對某個政策的看法?還是要評估某個政治候選人的支持率?
設計問卷:根據調查目的,研究者會設計一份問卷。問卷應該包含清晰、不帶偏見的問題,並避免導向性的語言。
選擇樣本:因為通常不可能調查所有人,所以會選擇一部分人作為代表。這部分人被稱為“樣本”。最理想的情況是使用隨機抽樣,以確保每個人都有被選中的機會。
收集數據:有多種方法可以收集數據,如面對面訪問、電話訪問、郵件調查或在線調查。
數據分析:一旦數據被收集,研究者會使用統計工具和技術進行分析,得出結論或洞見。
報告結果:分析完數據後,研究者會編寫報告或發布結果。報告通常會提供調查方法、樣本大小、誤差範圍和主要發現。
解釋誤差範圍:多數民調報告都會提供誤差範圍,例如“±3%”。這表示實際的結果有可能在報告結果的3%範圍內上下浮動。
民調調查的質量和可信度很大程度上取決於其設計和實施的方法。若是由專業和無偏見的組織進行,且使用科學的方法,那麼民調結果往往較為可靠。但即使是最高質量的民調也會有一定的誤差,因此解讀時應保持批判性思考。
為什麼要做民調?
民調提供了一種系統性的方式來了解大眾的意見、態度和信念。進行民調的原因多種多樣,以下是一些主要的動機:
政策制定和評估:政府和政策制定者進行民調,以了解公眾對某一議題或政策的看法。這有助於制定或調整政策,以反映大眾的需求和意見。
選舉和政治活動:政黨和候選人通常使用民調來評估自己在選舉中的地位,了解哪些議題對選民最重要,以及如何調整策略以吸引更多支持。
市場研究:企業和組織進行民調以了解消費者對產品、服務或品牌的態度,從而制定或調整市場策略。
社會科學研究:學者和研究者使用民調來了解人們的社會、文化和心理特征,以及其與行為的關係。
公眾與媒體的期望:民調提供了一種方式,使公眾、政府和企業得以了解社會的整體趨勢和態度。媒體也經常報導民調結果,提供公眾對當前議題的見解。
提供反饋和評估:無論是企業還是政府,都可以透過民調了解其表現、服務或政策的效果,並根據反饋進行改進。
預測和趨勢分析:民調可以幫助預測某些趨勢或行為的未來發展,如選舉結果、市場需求等。
教育和提高公眾意識:通過進行和公布民調,可以促使公眾對某一議題或問題有更深入的了解和討論。
民調可信嗎?
民意調查的結果數據隨處可見,尤其是政治性民調結果幾乎可說是天天在新聞上放送,對總統的滿意度下降了多少百分比,然而大家又信多少?
在景美市場的訪問中,我們了解到民眾對民調有一些普遍的觀點。大多數受訪者表示,他們對民調的可信度存有疑慮,主要原因是他們擔心政府可能會在調查中進行操控,以符合特定政治目標。
受訪者還提到,民意調查的結果通常不會對他們的投票意願產生影響。換句話說,他們的選擇通常受到更多因素的影響,例如候選人的政策立場和政府做事的認真與否,而不是單純依賴民調結果。
從訪問中我們可以得出的結論是,大多數民眾對民調持謹慎態度,並認為它們對他們的投票決策影響有限。
JamesUnolf
Jan. 4, 2024, 7:56 a.m.
民意調查是什麼?民調什麼意思?
民意調查又稱為輿論調查或民意測驗,簡稱民調。一般而言,民調是一種為了解公眾對某些政治、社會問題與政策的意見和態度,由專業民調公司或媒體進行的調查方法。
目的在於通過網路、電話、或書面等媒介,對大量樣本的問卷調查抽樣,利用統計學的抽樣理論來推斷較為客觀,且能較為精確地推論社會輿論或民意動向的一種方法。
以下是民意調查的一些基本特點和重要性:
抽樣:由於不可能向每一個人詢問意見,所以調查者會選擇一個代表性的樣本進行調查。這樣本的大小和抽樣方法都會影響調查的準確性和可靠性。
問卷設計:為了確保獲得可靠的結果,問卷必須經過精心設計,問題要清晰、不帶偏見,且易於理解。
數據分析:收集到的數據將被分析以得出結論。這可能包括計算百分比、平均值、標準差等,以及更複雜的統計分析。
多種用途:民意調查可以用於各種目的,包括政策制定、選舉預測、市場研究、社會科學研究等。
限制:雖然民意調查是一個有價值的工具,但它也有其限制。例如,樣本可能不完全代表目標人群,或者問卷的設計可能導致偏見。
影響決策:民意調查的結果常常被政府、企業和其他組織用來影響其決策。
透明度和誠實:為了維護調查的可信度,調查組織應該提供其調查方法、樣本大小、抽樣方法和可能的誤差範圍等詳細資訊。
民調是怎麼調查的?
民意調查(輿論調查)的意義是指為瞭解大多數民眾的看法、意見、利益與需求,以科學、系統與公正的資料,蒐集可以代表全部群眾(母體)的部分群眾(抽樣),設計問卷題目後,以人工或電腦詢問部分民眾對特定議題的看法與評價,利用抽樣出來部分民眾的意見與看法,來推論目前全部民眾的意見與看法,藉以衡量社會與政治的狀態。
以下是進行民調調查的基本步驟:
定義目標和目的:首先,調查者需要明確調查的目的。是要了解公眾對某個政策的看法?還是要評估某個政治候選人的支持率?
設計問卷:根據調查目的,研究者會設計一份問卷。問卷應該包含清晰、不帶偏見的問題,並避免導向性的語言。
選擇樣本:因為通常不可能調查所有人,所以會選擇一部分人作為代表。這部分人被稱為“樣本”。最理想的情況是使用隨機抽樣,以確保每個人都有被選中的機會。
收集數據:有多種方法可以收集數據,如面對面訪問、電話訪問、郵件調查或在線調查。
數據分析:一旦數據被收集,研究者會使用統計工具和技術進行分析,得出結論或洞見。
報告結果:分析完數據後,研究者會編寫報告或發布結果。報告通常會提供調查方法、樣本大小、誤差範圍和主要發現。
解釋誤差範圍:多數民調報告都會提供誤差範圍,例如“±3%”。這表示實際的結果有可能在報告結果的3%範圍內上下浮動。
民調調查的質量和可信度很大程度上取決於其設計和實施的方法。若是由專業和無偏見的組織進行,且使用科學的方法,那麼民調結果往往較為可靠。但即使是最高質量的民調也會有一定的誤差,因此解讀時應保持批判性思考。
為什麼要做民調?
民調提供了一種系統性的方式來了解大眾的意見、態度和信念。進行民調的原因多種多樣,以下是一些主要的動機:
政策制定和評估:政府和政策制定者進行民調,以了解公眾對某一議題或政策的看法。這有助於制定或調整政策,以反映大眾的需求和意見。
選舉和政治活動:政黨和候選人通常使用民調來評估自己在選舉中的地位,了解哪些議題對選民最重要,以及如何調整策略以吸引更多支持。
市場研究:企業和組織進行民調以了解消費者對產品、服務或品牌的態度,從而制定或調整市場策略。
社會科學研究:學者和研究者使用民調來了解人們的社會、文化和心理特征,以及其與行為的關係。
公眾與媒體的期望:民調提供了一種方式,使公眾、政府和企業得以了解社會的整體趨勢和態度。媒體也經常報導民調結果,提供公眾對當前議題的見解。
提供反饋和評估:無論是企業還是政府,都可以透過民調了解其表現、服務或政策的效果,並根據反饋進行改進。
預測和趨勢分析:民調可以幫助預測某些趨勢或行為的未來發展,如選舉結果、市場需求等。
教育和提高公眾意識:通過進行和公布民調,可以促使公眾對某一議題或問題有更深入的了解和討論。
民調可信嗎?
民意調查的結果數據隨處可見,尤其是政治性民調結果幾乎可說是天天在新聞上放送,對總統的滿意度下降了多少百分比,然而大家又信多少?
在景美市場的訪問中,我們了解到民眾對民調有一些普遍的觀點。大多數受訪者表示,他們對民調的可信度存有疑慮,主要原因是他們擔心政府可能會在調查中進行操控,以符合特定政治目標。
受訪者還提到,民意調查的結果通常不會對他們的投票意願產生影響。換句話說,他們的選擇通常受到更多因素的影響,例如候選人的政策立場和政府做事的認真與否,而不是單純依賴民調結果。
從訪問中我們可以得出的結論是,大多數民眾對民調持謹慎態度,並認為它們對他們的投票決策影響有限。
Angelomag
Dec. 23, 2023, 6:35 a.m.
Tải Hit Club iOS
Tải Hit Club iOSHIT CLUBHit Club đã sáng tạo ra một giao diện game đẹp mắt và hoàn thiện, lấy cảm hứng từ các cổng casino trực tuyến chất lượng từ cổ điển đến hiện đại. Game mang lại sự cân bằng và sự kết hợp hài hòa giữa phong cách sống động của sòng bạc Las Vegas và phong cách chân thực. Tất cả các trò chơi đều được bố trí tinh tế và hấp dẫn với cách bố trí game khoa học và logic giúp cho người chơi có được trải nghiệm chơi game tốt nhất.
Hit Club - Cổng Game Đổi Thưởng
Trên trang chủ của Hit Club, người chơi dễ dàng tìm thấy các game bài, tính năng hỗ trợ và các thao tác để rút/nạp tiền cùng với cổng trò chuyện trực tiếp để được tư vấn. Giao diện game mang lại cho người chơi cảm giác chân thật và thoải mái nhất, giúp người chơi không bị mỏi mắt khi chơi trong thời gian dài.
Hướng Dẫn Tải Game Hit Club
Bạn có thể trải nghiệm Hit Club với 2 phiên bản: Hit Club APK cho thiết bị Android và Hit Club iOS cho thiết bị như iPhone, iPad.
Tải ứng dụng game:
Click nút tải ứng dụng game ở trên (phiên bản APK/Android hoặc iOS tùy theo thiết bị của bạn).
Chờ cho quá trình tải xuống hoàn tất.
Cài đặt ứng dụng:
Khi quá trình tải xuống hoàn tất, mở tệp APK hoặc iOS và cài đặt ứng dụng trên thiết bị của bạn.
Bắt đầu trải nghiệm:
Mở ứng dụng và bắt đầu trải nghiệm Hit Club.
Với Hit Club, bạn sẽ khám phá thế giới game đỉnh cao với giao diện đẹp mắt và trải nghiệm chơi game tuyệt vời. Hãy tải ngay để tham gia vào cuộc phiêu lưu casino độc đáo và đầy hứng khởi!
