
NCERT Solutions for Class 12 Chemistry Chapter 13 Amines PDF
Hey, are you a class 12 student and looking for ways to download NCERT Solutions for Class 12 Chemistry Chapter 13 Amines PDF? If yes. Then read this post till the end.In this article, we have listed NCERT Solutions for Class 12 Chemistry Chapter 13 Amines in PDF that are prepared by Kota’s top IITian Faculties by keeping Simplicity in mind.
If you want to learn and understand class 12 Chemistry Chapter 13 "Amines" in an easy way then you can use these solutions PDF.
NCERT Solutions helps students to Practice important concepts of subjects easily. Class 12 Chemistry solutions provide detailed explanations of all the NCERT questions that students can use to clear their doubts instantly.
If you want to score high in your class 12 Chemistry Exam then it is very important for you to have a good knowledge of all the important topics, so to learn and practice those topics you can use eSaral NCERT Solutions.
In this article, we have listed NCERT Solutions for Class 12 Chemistry Chapter 13 Amines PDF that you can download to start your preparations anytime.
So, without wasting more time Let’s start.
Download NCERT Solutions for Class 12 Chemistry Chapter 13 Amines PDF
Question 1. Classify the following amines as primary, secondary or tertiary:
(i)
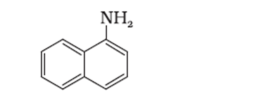
(ii)
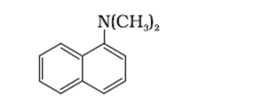
(iii) $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{CHNH}_{2}$
(iv) $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$
Solution. Primary: (i) and (iii)
$-\mathrm{N}$ atom is attached with one carbon or one alkyl group.
Secondary: (iv)
$-\mathrm{N}$ atom is attached with two carbons or two alkyl groups.
Tertiary: (ii)
−N group attached with three carbons or three alkyl group.
Question 2. (i) Write structures of different isomeric amines corresponding to the molecular formula, $\mathrm{C}_{4} \mathrm{H}_{11} \mathrm{~N}$.
(ii) Write IUPAC names of all the isomers.
(iii) What type of isomerism is exhibited by different pairs of amines?
Solution. (i), (ii): The structures and their IUPAC names of different isomeric amines corresponding to the molecular formula, $\mathrm{C}_{4} \mathrm{H}_{11} \mathrm{~N}$ is given below:
(a)
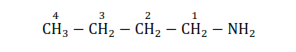
Parent carbon chain $=5$ membered (pent)
Primary suffix $=$ ane
Secondary suffix $=$ amine
IUPAC name: Butanamine (1^{\circ} ~ a m i n e ) ~
(b)
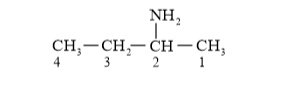
Parent carbon chain = 4 membered (but)
Primary suffix = ane
Secondary suffix = amine
IUPAC Name: Butan-2-amine $\left(1^{\circ}\right.$ amine $)$
(c)
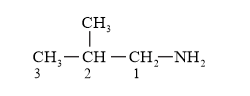
Parent carbon chain = 3 (prop)
Primary suffix = ane
Secondary suffix = amine
Substituent $=$ methyl $\left(-\mathrm{CH}_{3}\right)$
IUPAC Name: 2-Methypropanamine ( $1^{\circ}$ amine)
(d)
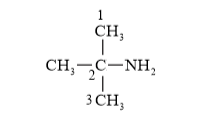
Parent carbon chain =3 (prop)
Primary suffix =ane
Secondary suffix = amine
Substituent $\left(\mathrm{CH}_{3}\right)=$ methyl
IUPAC Name: 2-Methylpropan-2-amine
(e) $\mathrm{CH}_{3}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{NH}-\mathrm{CH}_{3}$
IUPAC Name: N-Methylpropanamine ( $2^{\circ}$ amine)
(f) $\quad \mathrm{CH}_{3}-\mathrm{CH}_{2}^{3}-\mathrm{CH}_{2}-\mathrm{NH}-\mathrm{CH}_{3}$
IUPAC Name: N-Ethylethanamine ( $2^{\circ}$ amine)
(g)
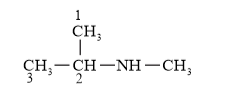
IUPAC Name: N-methylpropan -2-amine ( $2^{\circ}$ amine)
(h)
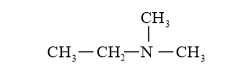
IUPAC Name: N, N-Dimenthylethanamine ( $3^{\circ}$ amine)
(iii) The pairs (a) and (b), (e) and (g) exhibit position isomerism because only the position of functional group $\left(-\mathrm{NH}_{2}\right)$ is changed.
The pairs (a) and (c), (a) and (d), (b) and (c), (b) and (d) exhibit chain isomerism because the parent carbon chains are different.
The pairs (e) and (f), (f) and (g) exhibit metamerism.
All primary amines exhibit functional isomerism with secondary and tertiary amines and vice-versa.
Question 3. How will you convert
(i) Benzene into aniline
(ii) Benzene into $\mathrm{N}, \mathrm{N}$-dimethylaniline
(iii) $\mathrm{Cl}-\left(\mathrm{CH}_{2}\right)_{4}-\mathrm{Cl}$ into hexan-1, 6 -diamine
Solution. (i)
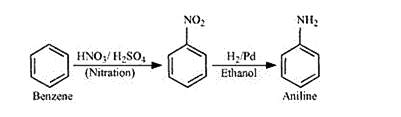
Reaction of Benzene with $\mathrm{HNO}_{3} / \mathrm{H}_{2} \mathrm{SO}_{4}$ is an electrophilic substitution reaction. $\mathrm{NO}_{2}^{+}$is a electrophile in the nitration reaction.
In the next step nitrobenzene is reduced by $\mathrm{H}_{2} / \mathrm{Pd}$ to aniline.
(ii)
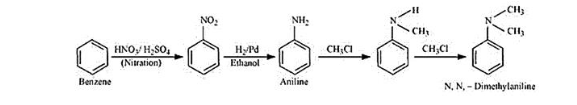
Reaction of Benzene with $\mathrm{HNO}_{3} / \mathrm{H}_{2} \mathrm{SO}_{4}$ is an electrophilic substitution reaction. $\mathrm{NO}_{2}^{+}$is a electrophile in the nitration reaction hence nitrobenzene formed.
In the next step(second), nitrobenzene is reduced by $\mathrm{H}_{2} / \mathrm{Pd}$ to aniline.
In the next two steps (third and fourth) the substitution reaction taking place and finally N,N-dimethylanilnine formed.
(iii)
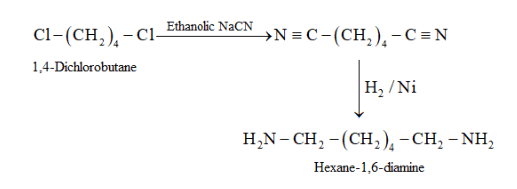
Substitution reaction takes place when 1,4 -dichlorobutane reacts with ethanolic $\mathrm{NaCN}$
In the next step, $-\mathrm{CN}$ group is reduced to form $1^{\circ}$ amine.
Question 4. Arrange the following in increasing order of their basic strength:
(i) $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}, \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}, \mathrm{NH}_{3}, \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{NH}_{2}$ and $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$
(ii) $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2},\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH},\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{3} \mathrm{~N}, \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}$
(iii) $\mathrm{CH}_{3} \mathrm{NH}_{2},\left(\mathrm{CH}_{3}\right)_{2} \mathrm{NH},\left(\mathrm{CH}_{3}\right)_{3} \mathrm{~N}, \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}, \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{NH}_{2}$.
Solution. (i) Considering +I effect of alkyl groups, $\mathrm{NH}_{3}, \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}$, and $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$ can be arranged in the increasing order of their basic strengths as:
$\mathrm{NH}_{3}<\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}<\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$
Again, in $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}$ acceptability of proton is less than $\mathrm{NH}_{3}$. Thus, we have:
Aromatic amine $<$ Aliphatic Amine (Basic character)
Also due to the $-$ I effective of $\mathrm{C}_{6} \mathrm{H}_{5}$ group, the electron density on the $\mathrm{N}$-atom in $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{NH}_{2}$ is lower than that on the $\mathrm{N}$-atom in $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}$,
but more than that in $\mathrm{NH}_{3}$. Therefore, the given compounds can be arranged in the order of their basic strengths as:
$\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}<\mathrm{NH}_{3}<\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{NH}_{2}<\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}<\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$ (overall basic strength)
(ii) Basic character $\propto+\mathrm{I}$ effect
Due to $+\mathrm{I}$ effect $-\mathrm{C}_{2} \mathrm{H}_{5}$ the basic character should be $\left.\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{3} \mathrm{~N}\right\rangle$ $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}>\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}$ in the gaseous phase. But in aqueous state hydrogen bonding is also taking place and extent of hydrogen bonding is $1^{\circ}>2^{\circ}>3^{\circ}$ amine
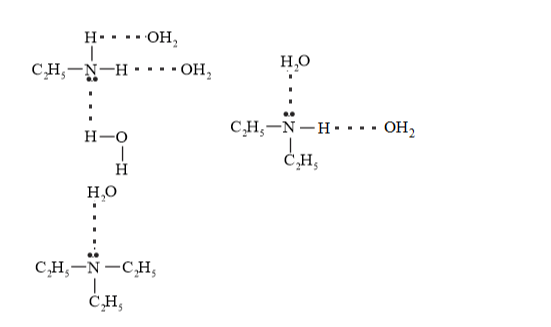
So overall basic character in aqueous medium is result of inductive effect and hydrogen bonding. So, order is $2^{\circ}>3^{\circ}>1^{\circ}$ amine (Aliphatic).
Considering the inductive effect and the steric hindrance of the alkyl groups in $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2},\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{3} \mathrm{~N}$ and $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$, their basic strengths, can be arranged as follows:
$\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}<\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{3} \mathrm{~N}<\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$
Again, due to the $-\mathrm{R}$ effect of $\mathrm{C}_{6} \mathrm{H}_{5}$ group, the electron density on the $\mathrm{N}$-atom in $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}$ is lower than that on the $\mathrm{N}$ atom in $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}$.
Therefore, the basicity of $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}$ is lower than that of $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}$ i.e., aromatic amine is less basic than aliphatic amine. Hence, the given compounds can be arranged in the increasing order of their basic strengths as follows:
$\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}<\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}<\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{3} \mathrm{~N}<\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$ (overall order)
(iii) Basic character $\propto+\mathrm{I}$
Considering the ( $+\mathrm{I}$ ) inductive effect and the steric hindrance of alkyl groups, $\mathrm{CH}_{3} \mathrm{NH}_{2},\left(\mathrm{CH}_{3}\right)_{2} \mathrm{NH}$, and $\left(\mathrm{CH}_{3}\right)_{3} \mathrm{~N}$ can be arranged in the increasing order of their basic strengths as:
$\left(\mathrm{CH}_{3}\right)_{3} \mathrm{~N}>\left(\mathrm{CH}_{3}\right)_{2} \mathrm{NH}>\left(\mathrm{CH}_{3}\right) \mathrm{NH}_{2}$ in gaseous phase.
In aqueous phase $\mathrm{H}$ - bonding is also taking place and extent of $\mathrm{H}$ - bond is $1^{\circ}>2^{\circ}>3^{\circ}$ Amine
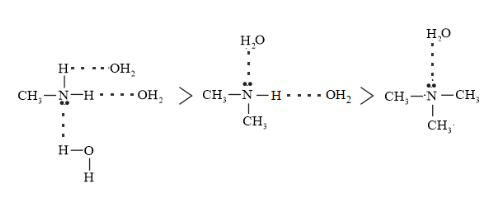
Resulting overall basic character in aqueous medium is due to inductive effect as well as hydrogen bonding so order of basic character is $2^{\circ}>$ $1^{\circ}>3^{\circ}$
In $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}, \mathrm{~N}$ is directly attached to the benzene ring. Thus, the lone pair of electrons on the $\mathrm{N}$-atom is delocalized over the benzene ring. In $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{NH}_{2}, \mathrm{~N}$ is not directly attached to the benzene ring. Thus, its lone pair is not delocalized over the benzene ring. Therefore, the electrons on the $\mathrm{N}$-atom are more easily available for protonation in $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{NH}_{2}$ than in $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}$
Again, due to the $-$ I effect of $\mathrm{C}_{6} \mathrm{H}_{5}$ group, the electron density on the $\mathrm{N}$-atom in $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{NH}_{2}$ is lower than that on the $\mathrm{N}$-atom in $\left(\mathrm{CH}_{3}\right)_{3} \mathrm{~N}$.
Therefore, $\left(\mathrm{CH}_{3}\right)_{3} \mathrm{~N}$ is more basic than $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{NH}_{2}$. i.e., Aromatic amines are less basic than aliphatic amine. Thus, the given compounds can be arranged in the increasing order of their basic strengths as follows.
$\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}<\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{NH}_{2}<\left(\mathrm{CH}_{3}\right)_{3} \mathrm{~N}<\mathrm{CH}_{3} \mathrm{NH}_{2}<\left(\mathrm{CH}_{3}\right)_{2} \mathrm{NH}$
Question 5. Complete the following acid-base reactions and name the products:
(i) $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{NH}_{2}+\mathrm{HCl} \rightarrow$
(ii) $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{3} \mathrm{~N}+\mathrm{HCl}$
Solution. n-propylamine is basic and HCl is acidic. So, acid-base reaction is taking place and salt is formed.
(i) $\underset{\text { n-Propylamine }}{\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{NH}_{2}}+\mathrm{HCl} \rightarrow \underset{\text { n-Propylammoniumchloride }}{\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{NH}_{3}^{+} \mathrm{Cl}^{-}}$
$\begin{aligned}&\text { (ii) }\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{3} \mathrm{~N}+\mathrm{HCl} \rightarrow & \left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{3} \mathrm{NH}_{3}^{+} \mathrm{Cl}^{-} \\&\text {Triethylamine } & \text { Triemethylammoniumchloride }\end{aligned}$
Triethylamine is basic and $\mathrm{HCl}$ is acidic. So, acid-base reaction is taking place and quaternary ammonium salt is formed.
Question 6. Write reactions of the final alkylation product of aniline with excess of methyl iodide in the presence of sodium carbonate solution.
Solution. Aniline on reaction with methyl iodide produces $\mathrm{N}, \mathrm{N}$-dimethylaniline by Hoffman degradation reaction.
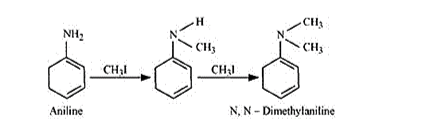
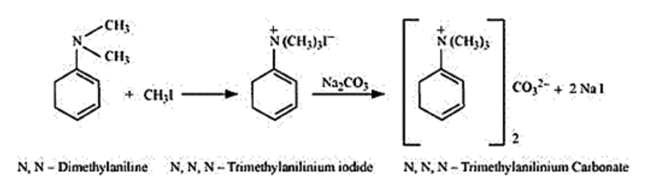
With excess methyl iodide, in the presence of $\mathrm{Na}_{2} \mathrm{CO}_{3}$ the solution, $\mathrm{N}, \mathrm{N}-$ dimethylaniline (quaternary ammonium salt) produces N, N, Ntrimethylanilinium carbonate.
Question 7. Write chemical reaction of aniline with benzoyl chloride and write the name of the product obtained.
Solution.

Question 8. Write structures of different isomers corresponding to the molecular formula, $\mathrm{C}_{3} \mathrm{H}_{9} \mathrm{~N}$. Write IUPAC names of the isomers which will liberate nitrogen gas on treatment with nitrous acid.
Solution: The structures of different isomers corresponding to the molecular formula, $\mathrm{C}_{3} \mathrm{H}_{9} \mathrm{~N}$ are given below:
(a) $\mathrm{CH}_{3}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{NH}_{2}$
Propan-1-amine
(b)
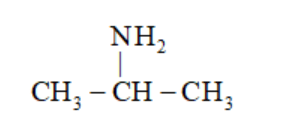
Propan-2-amine
(c) $\mathrm{CH}_{3}-\mathrm{NH}-\mathrm{C}_{2} \mathrm{H}_{5}$
$\mathrm{N}$ - Methylethanamine
(d)
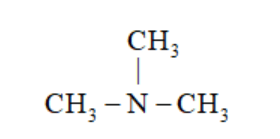
N, N – Dimethylmethanamine
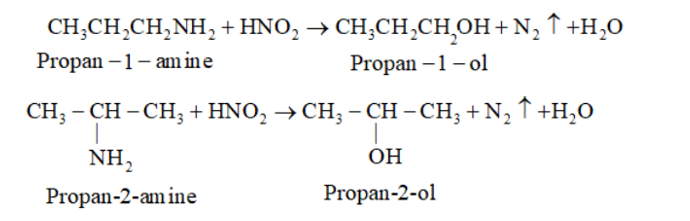
In (a) and (b), $-\mathrm{NH}_{2}$ group is attached to one $-\mathrm{C}$ atom. So, it is primary amine.
$\mathrm{R}-\mathrm{NH}_{2}($ Primary amine $)+\mathrm{HNO}_{2} \rightarrow \mathrm{R}-\mathrm{OH}+\mathrm{H}_{2} \mathrm{O}$
Nitrous acid $\left(\mathrm{HNO}_{2}\right)$ is a mono basic weak acid which is used to distinguish primary, secondary and tertiary amine.
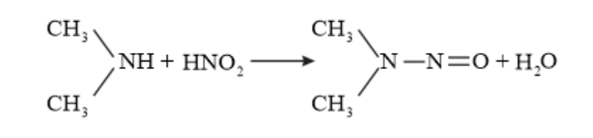
When secondary amines react with $\mathrm{HNO}_{2}$, no gas is produced, we get a yellow oil (nitrosamine).
Concept Insight: Primary aliphatic amines react with nitrous acid to form diazonium salt which being unstable liberate nitrogen gas.
Question 9. Convert
(i) $3-$ Methylaniline into $3-$ nitrotoluene.
c(ii) Aniline into $1,3,5-$ tribromobenzene.
Solution. (i)

(ii)

$\mathrm{NH}_{2}$ group is $+\mathrm{M}$ group. So, it is more activating group and ortho/para directing. Thus $\mathrm{Br}^{+}$attacks at ortho and para positions and forms trisubstituted bromo derivative compound. $2,4,6$-tribromoanline is white crystalline solid.
Question 10. Write IUPAC names of the following compounds and classify them into primary,
secondary and tertiary amines.
(i) $\left(\mathrm{CH}_{3}\right)_{2} \mathrm{CHNH}_{2}$
(ii) $\mathrm{CH}_{3}\left(\mathrm{CH}_{2}\right)_{2} \mathrm{NH}_{2}$
(iii) $\mathrm{CH}_{3} \mathrm{NHCH}\left(\mathrm{CH}_{3}\right)_{2}$
(iv) $\left(\mathrm{CH}_{3}\right)_{3} \mathrm{CNH}_{2}$
(v) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NHCH}_{3}$
(vi) $\left(\mathrm{CH}_{3} \mathrm{CH}_{2}\right)_{2} \mathrm{NCH}_{3}$
(vii) $\mathrm{m}-\mathrm{BrC}_{6} \mathrm{H}_{4} \mathrm{NH}_{2}$
Solution. (i) 1 -Methylethanamine $\left(1^{\circ}\right.$ amine $)$

(ii) Propan-1-amine ( $1^{\circ}$ amine)

(iii) $\mathrm{N}$-Methyl-2-methylethanamine ( $2^{0}$ amine $)$

(iv) 2 -Methylpropan-2-amine (1^{\circ} amine)

(v) $\mathrm{N}$-Methylbenzamine or $\mathrm{N}-$ methylaniline $\left(2^{\circ}\right.$ amine $)$

(vi) $\mathrm{N}$-Ethyl-N-methylethanamine $\left(3^{\circ}\right.$ amine $)$

(vii) 3 -Bromobenzenamine or 3 -bromoaniline $\left(1^{\circ}\right.$ amine $)$

Question 11. Give one chemical test to distinguish between the following pairs of compounds.
(i) Methylamine and dimethylamine
(ii) Secondary and tertiary amines
(iii) Ethylamine and aniline
(iv) Aniline and benzylamine
(v) Aniline and N-methylaniline.
Solution. (i) Carbylamine test is used to distinguish between Methylamine and dimethylamine.
Carbylamine test: Aliphatic and aromatic primary amines when heated with chloroform and ethanolic potassium hydroxide give foul-smelling isocyanides or carbylamines. Methylamine is an aliphatic primary amine that gives a positive carbylamine test, but dimethylamine does not give this reaction.
$\underset{\text { Methylamine }\left(1^{\circ}\right)}{\mathrm{CH}_{3}-\mathrm{NH}_{2}}+\mathrm{CHCl}_{3}+3 \mathrm{KOH} \stackrel{\Delta}{\rightarrow} \underset{\begin{array}{c}\text { Methyl isocyanide } \\ \text { (foul smell) }\end{array}}{\mathrm{CH}_{3}-\mathrm{NC}}+3 \mathrm{KCl}+3 \mathrm{H}_{2}$
$\left(\mathrm{CH}_{3}\right)_{2} \mathrm{NH}+\mathrm{CHCl}_{3}+3 \mathrm{KOH} \stackrel{\Delta}{\rightarrow}$ No reaction
(ii) Hinsberg's reagent (benzenesulphonyl chloride, $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{SO}_{2} \mathrm{Cl}$ ) is used to distinguish between secondary and tertiary amines.
Secondary amines on reaction with Hinsberg's reagent give a product which is insoluble in an alkali.
For example, $\mathrm{N}, \mathrm{N}-$ diethylamine on reaction with Hinsberg's reagent forms $\mathrm{N}, \mathrm{N}$-diethylbenzenesulphonamide, which is insoluble in an alkali. Whereas, tertiary amines do not react with Hinsberg's reagent because they do not have hydrogen.

(iii) Azo-dye test can be used to distinguish between ethylamine and aniline. When aromatic amines react with $\mathrm{HNO}_{2}\left(\mathrm{NaNO}_{2}+\right.$ dil. $\mathrm{HCl}$ ) at $0-5^{\circ} \mathrm{C}$, followed by a reaction with an alkaline solution of 2 -naphthol, a dye is formed. The dye formed is usually yellow, red or orange coloured.

In the above reaction, diazotisation takes place.

At $\mathrm{pH} 9-10$ in the presence of base phenol and diazonium salt, coupling reaction takes place and forms an azo dye.
Aliphatic amines give a brisk effervescence under similar conditions due to the evolution of $\mathrm{N}_{2}$ gas.
$\mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{NH}_{2}+\mathrm{HONO} \stackrel{0-5^{\circ} \mathrm{C}}{\rightarrow} \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}+\mathrm{N}_{2} \uparrow+\mathrm{H}_{2} \mathrm{O}$
(iv) Nitrous acid can be used to distinguish aniline and benzylamine. Nitrous acid is prepared in situ from a mineral acid and sodium nitrate. Benzylamine on reaction with nitrous acid forms an unstable diazonium salt. The unstable diazonium salt gives alcohol with the evolution of nitrogen gas.

On the other hand, aniline reacts with nitrous acid at a low temperature to form a stable diazonium salt. Nitrogen gas is not evolved in this case.
$\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2} \frac{\mathrm{NaNO}_{2}+\mathrm{HCl}}{273-278} \longrightarrow \mathrm{C}_{6} \mathrm{H}_{5}-\underset{\text { Diazonium salt(stable })}{\mathrm{N}_{2}^{+} \mathrm{Cl}^{-}+\mathrm{NaCl}+2 \mathrm{H}_{2} \mathrm{O}}$
(v) Carbylamine test is used to distinguish between aniline and $\mathrm{N}$-methylaniline. Primary amines, when heated with chloroform and ethanolic potassium hydroxide, form foul-smelling isocyanides or carbylamines. Aniline gives positive Carbylamine test because it is a primary aromatic amine. However, $N$-methylaniline (secondary amine) does not.

Question 12. Account for the following:
(i) $\mathrm{pK}_{\mathrm{b}}$ of aniline is more than that of methylamine.
(i) $\mathrm{pK}_{\mathrm{b}}$ of aniline is more than that of methylamine.
(iii) Methylamine in water reacts with ferric chloride to precipitate hydrated ferric oxide.
(iv) Although amino group is 0, and $p$ - directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of $m$-nitroaniline.
(v) Aniline does not undergo Friedel-Crafts reaction.
(vi) Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
(vii) Gabriel phthalimide synthesis is preferred for synthesising primary amines.
Solution. (i) $\mathrm{pK}_{\mathrm{b}}=-\log \mathrm{k}_{\mathrm{b}}$
If the basic character is more then $\mathrm{k}_{\mathrm{b}}$ value is high and $\mathrm{pK}_{\mathrm{b}}$ value is less.

Aniline undergoes resonance. So, the electrons on the nitrogen atom are delocalised over the benzene ring. Therefore, the electrons on the nitrogen atom are less available to donate. So, the basic character is less.

In methylamine, due to the +I effect of the methyl group (electron-donating group), the electron density on the $\mathrm{N}$-atom increases and lone pair of nitrogen is not involved in delocalisation. Thus, the basic character increases.

$\mathrm{K}_{\mathrm{b}}$ value

$\mathrm{pK}_{\mathrm{b}}$ value

(ii) Ethylamine, on addition with water, forms intermolecular H -bonds with water. Hence, it is soluble in water.

But aniline does not form H - bonds with water to a very large extent due to the presence of a large hydrophobic $-\mathrm{C}_{6} \mathrm{H}_{5}$ group. Hence, aniline is insoluble in water.

(iii)

Amines are more basic than water because the lone pair donor atom in amine is nitrogen and in water is oxygen. And nitrogen is less electronegative atom than oxygen.N can easily donate lone pair and $+\mathrm{I}$ effect of $-\mathrm{CH}_{3}$ group, methylamine is more basic than water. Therefore, in water, methylamine produces $\mathrm{OH}^{-}$ions by accepting $\mathrm{H}^{+}$ions from water.

Ferric chloride $\left(\mathrm{FeCl}_{3}\right)$ dissociates in water to form $\mathrm{Fe}^{3+}$ and $\mathrm{Cl}^{-}$ions.
$\mathrm{FeCl}_{3} \rightarrow \mathrm{Fe}^{3+}+3 \mathrm{Cl}^{-}$
Then, $\mathrm{OH}^{-}$ion reacts with $\mathrm{Fe}^{3+}$ ion and forms a precipitate of hydrated ferric oxide.
$2 \mathrm{Fe}^{3+}+6 \mathrm{OH}^{-} \rightarrow \underset{\text { Hydrated ferric oxide }(\mathrm{ppt})}{\mathrm{Fe}_{2} \mathrm{O}_{3} .3 \mathrm{H}_{2} \mathrm{O}}$
(iv) Nitration is carried out in an acidic medium. In an acidic medium, the aniline is protonated to give anilinium ion. $+\mathrm{NH}_{3}$ group shows $-\mathrm{I}$ effect (electron-withdrawing group). So, it is meta directing.

(v) Aniline does not undergo Friedel-crafts reaction:
Friedel-Crafts reaction takes place in the presence of $\mathrm{AlCl}_{3}$. But $\mathrm{AlCl}_{3}$ is a lewis acid due to vacant orbital availability while aniline is a strong base. So, an acid-base reaction will take place. Thus, aniline reacts with $\mathrm{AlCl}_{3}$ to form a salt.

Due to the positive charge on the nitrogen atom (shows $-I$ effect and it is meta directing), electrophilic substitution in the benzene ring is deactivated. Hence, the Friedel-Crafts reaction will not take place in aniline.
(vi) The aromatic diazonium ion undergoes resonance.

This resonance is the reason for the stability of the diazonium ion. While in aliphatic amine no delocalisation takes place. Hence, the diazonium salts of aromatic amines are more stable than those of aliphatic amines.
(vii) Only $1^{\circ}$ amine is formed by Gabriel phthalimide synthesis. $2^{\circ}$ and $3^{\circ}$ amines are not formed in this synthesis. So, a pure $1^{\circ}$ amine can be obtained. Therefore Gabriel phthalimide synthesis is preferred for synthesizing primary amines.

Question 13. Arrange the following:
(i) In decreasing order of the $\mathrm{pK}_{\mathrm{b}}$ values:
$\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}, \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NHCH}_{3},\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}$
(ii) In increasing order of basic strength:
$\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}, \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{~N}\left(\mathrm{CH}_{3}\right)_{2},\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$ and $\mathrm{CH}_{3} \mathrm{NH}_{2}$
(iii) In increasing order of basic strength:
(a) Aniline, p -nitroaniline and p -toluidine
(b) $\quad \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}, \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NHCH}_{3}, \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{NH}_{2}$.
(iv) In decreasing order of the basic strength in the gas phase:
$\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2},\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH},\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{3} \mathrm{~N}$ and $\mathrm{NH}_{3}$
(v) In increasing order of boiling point:
$\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH},\left(\mathrm{CH}_{3}\right)_{2} \mathrm{NH}, \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}$
(vi) In increasing order of solubility in water:
$\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2},\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}, \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2} .$
Solution. (i) In decreasing order of the $\mathrm{pK}_{\mathrm{b}}$ values:
$\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}, \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NHCH}_{3},\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}$
$\mathrm{pK}_{\mathrm{b}}=-\log \mathrm{K}_{\mathrm{b}}$
If $\mathrm{pK}_{\mathrm{b}}$ value is less, then more the value of $\mathrm{K}_{\mathrm{b}}$ and the basic character.
$\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NHCH}_{3}$ are aromatic amine while $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$ and
$\mathrm{CH}_{3}-\mathrm{NH}_{2}$ are aliphatic amines.
$-\mathrm{C}_{2} \mathrm{H}_{5}$ group is showing $+\mathrm{I}$ effect
In $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}$, only one $-\mathrm{C}_{2} \mathrm{H}_{5}$ group is present while in $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$, two
$-\mathrm{C}_{2} \mathrm{H}_{5}$ groups are present. Thus, the $+\mathrm{I}$ effect more in $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2}$ than in $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}$. Therefore, the electron density over the $\mathrm{N}$-atom is more in $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$ than in $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}$. Hence, $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$ is more basic than
$\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}$. Both $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NHCH}_{3}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}$ are less basic than $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$
and $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}$ due to the delocalization of the lone pair in the former two. Further, among $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NHCH}_{3}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}$, the former will be more basic due to the $+$ I effect of $-\mathrm{CH}_{3}$ group.
$\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}<\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NHCH}_{3}$ (basic character)
The basic character of aromatic amine is less than aliphatic amine because lone pair of nitrogen in aromatic amine is involved in delocalisation while in aliphatic amine it is not involved in delocalisation hence overall basic character is $\left(\mathrm{K}_{\mathrm{b}}\right)$
$\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}<\mathrm{C}_{6} \mathrm{H}_{5}-\mathrm{NHCH}_{3}<\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}<\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$
Hence the order of $\mathrm{pK}_{\mathrm{b}}$ is
$\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}>\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NHCH}_{3}>\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}>\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$
$\because \mathrm{pK}_{\mathrm{b}}=-\log \mathrm{K}_{\mathrm{b}}$
We know that the higher the basic strength, the lower is the $\mathrm{pK}_{\mathrm{b}}$ values.
$\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}>\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NHCH}_{3}>\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}>\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$
(ii) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{~N}\left(\mathrm{CH}_{3}\right)$ is more basic than $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}$ due to the $+\mathrm{I}$ effect of two
$-\mathrm{CH}_{3}$ groups in $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{~N}\left(\mathrm{CH}_{3}\right)_{2} .$ Also, $\mathrm{CH}_{3} \mathrm{NH}_{2}$ contains one $-\mathrm{CH}_{3}$ group
while $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$ contains two $-\mathrm{C}_{2} \mathrm{H}_{5}$ groups. Thus, $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$ is more basic than $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}$.
Now, $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{~N}\left(\mathrm{CH}_{3}\right)_{2}$ is less basic than $\mathrm{CH}_{3} \mathrm{NH}_{2}$ because of the $-\mathrm{R}$ effect of $-\mathrm{C}_{6} \mathrm{H}_{5}$ group.
Hence, the increasing order of the basic strength of the given compounds is as follows:
$\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}<\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{~N}\left(\mathrm{CH}_{3}\right)_{2}<\mathrm{CH}_{3} \mathrm{NH}_{2}<\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$
(iii) (a)

In p -substituted amine, the presence of electron-donating $-\mathrm{CH}_{3}$ group $(+\mathrm{H}>+\mathrm{I})$ increases the electron density on the nitrogen atom. Hence, the basic character increases.
Thus, $p$-toluidine is more basic than aniline.
On the other hand, due to the presence of electron-withdrawing $-\mathrm{NO}_{2}$ group $(-\mathrm{M}>-\mathrm{I})$ decreases the electron density over the $\mathrm{N}$-atom in $\mathrm{p}$-nitroaniline. Thus, $\mathrm{p}$-nitroaniline is less basic than aniline.
Hence, the increasing order of the basic strengths of the given compounds is as follows:

(b) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NHCH}_{3}$ is more basic than $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}$ due to the presence of
electron-donating $-\mathrm{CH}_{3}$ group $(+H>+I$ inductive effect) in $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NHCH}_{3} .$
Again, in $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NHCH}_{3},-\mathrm{C}_{6} \mathrm{H}_{5}$ group is directly attached to the nitrogen atom. However, it is not so in $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{NH}_{2}$. The lone pair of nitrogen is not involved in delocalisation. Thus, in $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NHCH}_{3}$, the $-\mathrm{R}$ effect of $-\mathrm{C}_{6} \mathrm{H}_{5}$ group decreases the electron density over the nitrogen atom. Therefore, $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{NH}_{2}$ is more basic than $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NHCH}_{3} .$
Hence, the increasing order of the basic strength of the given compounds is as follows:

(iv) The basic strength mainly depends upon the electron-donating group (+I effect) because there is no solvation effect in the gas phase. More is the $+\mathrm{I}$ effect, stronger is the base. Also, the more the number of alkyl groups, the higher is the $+\mathrm{I}$ effect. Therefore, the given compounds can be arranged in the decreasing order of their basic strengths in the gas phase as follows:
$\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{3} \mathrm{~N}>\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}>\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}>\mathrm{NH}_{3}$
(v) The boiling points of compounds depend on the hydrogen bonding present in the compound. The more extensive the $\mathrm{H}$-bonding in the compound, the higher is the boiling point. $\left(\mathrm{CH}_{3}\right)_{2} \mathrm{NH}$ contains only one $\mathrm{H}-$ atom whereas $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}$ contains two $\mathrm{H}$-bonding than $\left(\mathrm{CH}_{3}\right)_{2} \mathrm{NH} .$ Hence, the boiling point of $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}$ is higher than that of $\left(\mathrm{CH}_{3}\right)_{2} \mathrm{NH}$.
Boiling point $\propto$ force of attraction $\propto \mathrm{H}$ - bond
Force of attraction between $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}$ is also H-bond. $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}$ forms stronger $\mathrm{H}$-bonds than $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}$ and $\left(\mathrm{CH}_{3}\right)_{2} \mathrm{NH} .$


The given compounds can be arranged in the increasing order of their boiling points as follows:
$\left(\mathrm{CH}_{3}\right)_{2} \mathrm{NH}<\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}<\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}$
(vi) Solubility is directly proportional to the extent of hydrogen bonding. $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}$ contains two $\mathrm{H}$-atoms whereas $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$ contains only one $\mathrm{H}$-atom. Thus, $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}$ undergoes more extensive H - bonding than $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH} .$ Hence, the solubility in water of $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}$ is more than that of $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH} .$
Further, the solubility of amines decreases with an increase in the molecular mass. This is because the molecular mass of amines increases with an increase in the size of the hydrophobic part. The molecular mass of $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}$ is greater than that of $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}$ and $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH} .$ Also due to steric crowding the extent of hydrogen bonding in $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}$ decreases.


Hence, the increasing order of their solubility in water is as follows:
$\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}<\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}<\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}$
Question 14. How will you convert:
(i) Ethanoic acid into methanamine
(ii) Hexanenitrile into 1 −aminopentane
(iii) Methanol to ethanoic acid
(iv) Ethanamine into methanamine
(v) Ethanoic acid into propanoic acid
(vi) Methanamine into ethanamine
(viii) Propanoic acid into ethanoic acid?
Solution. (i)

(ii)

(iii)

(iv)

(v)

(vi)

(vii)

(viii)

Question 15. Describe a method for the identification of primary, secondary and tertiary amines. Also, write chemical equations of the reactions involved.
Solution. Hinsberg's test is used to identify and distinguish primary, secondary and tertiary amines. In the Hinsberg's test, the amines are allowed to react with Hinsberg's reagent, benzenesulphonyl chloride $\left(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{SO}_{2} \mathrm{Cl}\right) .$ Different types of amines react differently with the Hinsberg's reagent.
Primary amines react with benzenesulphonyl chloride to form N - alkylbenzenesulphonyl amide which is soluble in alkali.

Due to the presence of a strong electron-withdrawing sulphonyl group in the sulphonamide, the H -atom attached to nitrogen can be easily released as a proton. Therefore, it is acidic and dissolves in an alkali.
Secondary amines react with Hinsberg's reagent to give a sulphonamide which is insoluble in alkali.

There is no $\mathrm{H}$-atom attached to the $\mathrm{N}$-atom in the sulphonamide. Therefore, it is not acidic and is insoluble in an alkali.
Tertiary amines do not react with Hinsberg's reagent at all.
Question 16. Write short notes on the following:
(i) Carbylamine reaction
(ii) Diazotisation
(iii) Hofmann’s bromamide reaction
(iv) Coupling reaction
(v) Ammonolysis
(vi) Acetylation
(vii) Gabriel phthalimide synthesis.
Solution. (i) Carbylamine reaction
Carbylamine reaction is used as a test for the identification of primary amines. When aliphatic and aromatic primary amines are heated with chloroform $\left(\mathrm{CHCl}_{3}\right)$ and ethanolic potassium hydroxide (KOH), carbylamines (or isocyanides) are formed. These carbylamines have very unpleasant odours. Secondary and tertiary amines do not respond to this test.
General reaction:

Where $\mathrm{R}=\mathrm{Alkyl}$ group
- R Should not be $-\mathrm{CH}_{3}$ because $\mathrm{CH}_{3}-\mathrm{NC}$ (Methyl isocyanide) is formed, which is a poisonous gas.
For example,

(ii) Diazotisation
Aliphatic or Aromatic primary amines react with nitrous acid (prepared in situ from $\mathrm{NaNO}_{2}$ and a mineral acid such as $\mathrm{HCl}$ ) at low temperatures $(273-278 \mathrm{~K})$ to form diazonium salts. The above conversion of primary aromatic amines into diazonium salts is known as diazotisation.
For example, on treatment with $\mathrm{NaNO}_{2}$ and $\mathrm{HCl}$ at $273-278 \mathrm{~K}$, aniline produces the compound benzene diazonium chloride, with $\mathrm{NaCl}$ and $\mathrm{H}_{2} \mathrm{O}$ as by-products.


(iii) Hoffmann bromamide reaction
When an amide is treated with bromine in an aqueous or ethanolic solution of sodium hydroxide, a primary amine with one carbon atom less than the original amide is produced. This degradation reaction is known as Hoffmann bromamide reaction. This reaction involves the migration of an alkyl or aryl group from the carbonyl carbon atom of the amide to the nitrogen atom.
General reaction

Reaction Mechanism:
$\begin{aligned} &+-\\ \mathrm{KOH}+\mathrm{Br}_{2} \rightarrow & \mathrm{KOBr} \end{aligned}$

For example,


(iv) Coupling reaction:
The reaction in which two aromatic rings are joined through the $-N=$ $N$ - bond is known as coupling reaction. Arenediazonium salts such as benzene diazonium salts react with phenol or aromatic amines to form coloured azo compounds.

Dil $\mathrm{NaOH}$ at $p H=8.5$ to $9.5$ reacts with $-H$ of phenol group. Due to this phenoxide ion is formed. It activates the ring. When the phenoxide ion reacts with a diazonium salt, it results in the formation of azo dye. This reaction is called coupling reaction.

It is observed that the para-positions of phenol and aniline are coupled with the diazonium salt. This reaction proceeds through electrophilic substitution.
(v) Ammonolysis
When an alkyl or benzyl halide is reacted with an ethanolic solution of ammonia, it undergoes nucleophilic substitution reaction in which the halogen atom is replaced by an amino group $\left(-\mathrm{NH}_{2}\right)$. This process of cleavage of the carbon-halogen bond is known as ammonolysis.

This substituted ammonium salt when treated with a strong base such as sodium hydroxide, amine is obtained.

Though primary amine an as the major product, this process produces a mixture of primary, secondary and tertiary amines, and also a quaternary ammonium salt as shown.

(vi) Acetylation
Acetylation (or ethanoylation) is the process of introducing an acetyl group into a molecule.

Aliphatic and aromatic primary and secondary amines undergo acetylation reaction by nucleophilic substitution when treated with acid chlorides, anhydrides or esters. This reaction involves the replacement of the hydrogen atom of $-\mathrm{NH}_{2}$ or $>$ NH group by the acetyl group, which in turn leads to the production of amides. To shift the equilibrium to the right-hand side, the HCl formed during the reaction is removed as soon as it is formed. This reaction is carried out in the presence of a base (such as pyridine) which is stronger than the amine.


When amines react with benzoyl chloride, the reaction is also known as benzoylation. And this is followed by an electrophile substitution reaction
For example,

(vii) Gabriel phthalimide synthesis
Gabriel phthalimide synthesis is a very useful method for the preparation of aliphatic primary amines. It involves the treatment of phthalimide with ethanolic potassium hydroxide to form potassium salt of phthalimide. This salt is further heated with an alkyl halide, followed by alkaline hydrolysis to yield the corresponding primary amine.

Question 17. Accomplish the following conversions:
(i) Nitrobenzene to benzoic acid
(ii) Benzene to m-bromophenol
(iii) Benzoic acid to aniline
(iv) Aniline to 2,4,6 −tribromofluorobenzene
(v) Benzyl chloride to 2 −phenylethanamine
(vi) Chlorobenzene to 𝑝 −chloroaniline
(vii) Aniline to p-bromoaniline
(viii) Benzamide to toluene
(ix) Aniline to benzyl alcohol.
Solution. (i) Nitrobenzene to benzoic acid:

(ii)

(iii)

(iv)

(v)

(vi)



(vii)

(viii)

(ix)

Question 18. Give the structures of A, B and C in the following reactions:
(i) $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{I} \stackrel{\mathrm{NaCN}}{\longrightarrow} \mathrm{A} \frac{\mathrm{OH}^{-}}{\text {Parual hydrolysts }}{\longrightarrow} \mathrm{B} \stackrel{\mathrm{NaOH}+\mathrm{Br}_{2}}{\longrightarrow} \mathrm{C}$
(ii) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{~N}_{2} \mathrm{Cl} \stackrel{\mathrm{CuCN}}{\longrightarrow} \mathrm{A} \stackrel{\mathrm{H}_{2} \mathrm{O} / \mathrm{H}^{+}}{\longrightarrow} \mathrm{B} \frac{\mathrm{NH}_{3}}{\Delta} \longrightarrow \mathrm{C}$
(iii) $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{Br} \stackrel{\mathrm{KCN}}{\longrightarrow} \mathrm{A} \stackrel{\mathrm{LAH}_{4}}{\longrightarrow} \mathrm{B} \frac{\mathrm{HNO}_{2}}{\mathrm{O}^{\circ} \mathrm{C}} \longrightarrow \mathrm{C}$
(iv) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NO}_{2} \stackrel{\mathrm{Fe} / \mathrm{HCl}}{\longrightarrow} \mathrm{A} \frac{\mathrm{NaNO}_{2}+\mathrm{HCl}}{273 \mathrm{~K}} \longrightarrow \mathrm{B} \frac{\mathrm{H}_{2} \mathrm{O} / \mathrm{H}^{*}}{\Delta} \longrightarrow \mathrm{C}$
(v) $\mathrm{CH}_{3} \mathrm{COOH} \frac{\mathrm{NH}_{3}}{\Delta} \rightarrow \mathrm{A} \stackrel{\mathrm{NaOBr}}{\longrightarrow} \mathrm{B} \stackrel{\mathrm{NaNO}_{2} / \mathrm{HCl}}{\longrightarrow} \mathrm{C}$
(vi) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NO}_{2} \stackrel{\mathrm{Fe} / \mathrm{HCl}}{\longrightarrow} \mathrm{A}-\frac{\mathrm{HNO}_{3}}{273 \mathrm{~K}} \longrightarrow \mathrm{B} \stackrel{\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{OH}}{\longrightarrow} \mathrm{C}$
Solution: (i)

(ii)

(iii)

(iv)

(v)

(vi)

Question 19. An aromatic compound 'A' on treatment with aqueous ammonia and heating forms compound 'B' which on heating with $\mathrm{Br}_{2}$ and $\mathrm{KOH}$ forms a compound 'C' of molecular formula $\mathrm{C}_{6} \mathrm{H}_{7} \mathrm{~N}$. Write the structures and IUPAC names of compounds A, B and C.
Solution. It is given in the question the compound ' $\mathrm{C}$ ' having the molecular formula, $\mathrm{C}_{6} \mathrm{H}_{7} \mathrm{~N}$ is formed by heating compound 'B' with $\mathrm{Br}_{2}$ and $\mathrm{KOH}$. Reaction with $\mathrm{Br}_{2}$ or $\mathrm{KOH}$ is a Hoffman bromamide degradation reaction and is given by an amide. The formed product is an amine (one carbon down amide). Therefore, compound 'B' is an amide and compound ' $\mathrm{C}$ ' is an amine. The only amine having the molecular formula, $\mathrm{C}_{6} \mathrm{H}_{7} \mathrm{~N}$ is aniline, $\left(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}\right)$.
Therefore, compound 'B' must be benzamide, $\left(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CONH}_{2}\right)$
Further, benzamide is formed by heating compound 'A' with aqueous ammonia. Therefore, compound 'A' must be benzoic acid.
The given reaction occurs in the following sequence:

Question 20. Complete the following reactions:
(i) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}+\mathrm{CHCl}_{3}+$ alc. $\mathrm{KOH} \rightarrow$
(ii) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{~N}_{2} \mathrm{Cl}+\mathrm{H}_{3} \mathrm{PO}_{2}+\mathrm{H}_{2} \mathrm{O} \rightarrow$
(iii) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}+\mathrm{H}_{2} \mathrm{SO}_{4}$ (conc. $) \rightarrow$
(iv) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{~N}_{2} \mathrm{Cl}+\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH} \rightarrow$
(v) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}+\mathrm{Br}_{2}(\mathrm{aq}) \rightarrow$
(vi) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}+\left(\mathrm{CH}_{3} \mathrm{CO}\right)_{2} \mathrm{O} \rightarrow$
Solution:(i)

(ii)

The above reaction is a redox reaction.
(iii)

$-\mathrm{NH}_{2}$ group is basic and $\mathrm{H}_{2} \mathrm{SO}_{4}$ is acid. Hence salt is formed by an acidbase reaction.
(iv)

This reaction is a redox reaction.
(v)

An aniline $-N H_{2}$ is $+M$ group and it is a ring activating group for electrophilic attack and ortho-para directory. So, electrophile $-N O_{2}^{+}$attack at ortho-para. $2,4,6$-Tribromo aniline is formed, which is a white crystalline solid.
(vi)

(vii)

Question 21. Why cannot aromatic primary amines be prepared by Gabriel phthalimide synthesis?
Solution. Gabriel phthalimide synthesis is used for the preparation of aliphatic primary amines. It involves nucleophilic substitution $\left(\mathrm{S}_{\mathrm{N}} 2\right)$ of alkyl halides by the phthalimide.

But aryl halides do not undergo nucleophilic substitution with the anion formed by the phthalimide because positive charge on the benzene ring is highly unstable due to the double bond character.

Hence, primary aromatic amines cannot be prepared by this process.
Question 22. Write the reactions of
(i) aromatic amines with nitrous acid
(ii) aliphatic primary amines with nitrous acid.
Solution. (i) Aromatic amines react with nitrous acid (prepared in situ from $\mathrm{NaNO}_{2}$ and a mineral acid such as $\mathrm{HCl}$ ) at $273-278 \mathrm{~K}$ to form stable aromatic diazonium salts due to the double bond character.

(ii) Aliphatic primary amines react with nitrous acid (prepared in situ from $\mathrm{NaNO}_{2}$ and a mineral acid such as $\mathrm{HCl}$ ) to form unstable aliphatic diazonium salts, which further produce alcohol and $\mathrm{HCl}$ with the evolution of $\mathrm{N}_{2}$ gas.

Question 23. Give plausible explanation for each of the following:
(i) Why are amines less acidic than alcohols of comparable molecular masses?
(ii) Why do primary amines have higher boiling point than tertiary amines?
(iii) Why are aliphatic amines stronger bases than aromatic amines?
Solution. (i) Amines undergo protonation to give amide ion.

Similarly, alcohol loses a proton to give alkoxide ion.

Acidic strength ∝ stability of conjugate base or anion
Stability of conjugate base or anion $\propto$ negative charge density on anion $\propto$ electronegativity of atom
In an amide, ion, the negative charge is on the N-atom, whereas, in an alkoxide ion, the negative charge is on the O-atom. Since $\mathrm{O}$ is more electronegative than $\mathrm{N}, \mathrm{O}$ can accommodate the negative charge more easily than N. As a result, the amide ion is less stable than the alkoxide ion i.e., the conjugate base amide is less stable then alkoxide ion. Hence, amines are less acidic than alcohols of comparable molecular masses.
(ii) In a molecule of a tertiary amine, there are no H-atoms. Whereas, in the primary amines, two hydrogen atoms are present. Due to the presence of $\mathrm{H}$ atoms, primary amines undergo extensive intermolecular H-bonding.


Very less hydrogen bonding in tertiary amine due to steric hindrance of alkyl group (-R).
To break the hydrogen bond, extra energy is required to separate the molecules of primary amines. Hence, primary amines have higher boilingpoints than tertiary amines.
(iii) Due to the -R effect of the benzene ring, the electrons on the N-atom are less available in case of aromatic amines. Therefore, the electrons on the N-atom in aromatic amines cannot be donated easily. This explains why aliphatic amines are stronger bases than aromatic amines.
Aromatic amine:

For aliphatic amine $\mathrm{R} \rightarrow \mathrm{NH}_{2}, \mathrm{R}$ exerts $+\mathrm{I}$ effect. Charge density on nitrogen increases; hence, basic character increases.
Also Read,
Download Class 12 Chemistry Notes Free PDF.
Download Class 12 Chemistry Book Chapterwise Free PDF.
Download Class 12 Chemistry Exemplar Chapterwise Free PDF.
If you have any Confusion related to NCERT Solutions for Class 12 Chemistry Chapter 13 Amines PDF then feel free to ask in the comments section down below.
To watch Free Learning Videos on Class 12 Chemistry by Kota’s top IITan’s Faculties Install the eSaral App
