
NCERT Solutions for Class 12 Chemistry Chapter 3 Electrochemistry PDF
Hey, are you a class 12 student and looking for ways to download NCERT Solutions for Class 12 Chemistry Chapter 3 Electrochemistry PDF? If yes. Then read this post till the end.In this article, we have listed NCERT Solutions for Class 12 Chemistry Chapter 3 Electrochemistry in PDF that are prepared by Kota’s top IITian Faculties by keeping Simplicity in mind.
If you want to learn and understand class 12 Chemistry Chapter 3 "Electrochemistry" in an easy way then you can use these solutions PDF.
NCERT Solutions helps students to Practice important concepts of subjects easily. Class 12 Chemistry solutions provide detailed explanations of all the NCERT questions that students can use to clear their doubts instantly.
If you want to score high in your class 12 Chemistry Exam then it is very important for you to have a good knowledge of all the important topics, so to learn and practice those topics you can use eSaral NCERT Solutions.
In this article, we have listed NCERT Solutions for Class 12 Chemistry Chapter 3 Electrochemistry PDF that you can download to start your preparations anytime.
So, without wasting more time Let’s start.
Download NCERT Solutions for Class 12 Chemistry Chapter 3 Electrochemistry PDF
Question 1. How would you determine the standard electrode potential of the system $\mathrm{Mg}^{2+} \mid \mathrm{Mg}$ ?
Solution. The standard electrode potential of $\mathrm{Mg}^{2+} \mid \mathrm{Mg}$ can be measured with respect to the standard hydrogen electrode, represented by $\mathrm{Pt}(\mathrm{s}), \mathrm{H}_{2}(\mathrm{~g})(1 \mathrm{~atm}) \mid \mathrm{H}^{+}($aq. $)(1 \mathrm{M})$.
A cell, consisting of $\mathrm{Mg} \mid \mathrm{MgSO}_{4}(\mathrm{aq} 1 \mathrm{M})$ as the anode
$\mathrm{Mg} \rightarrow \mathrm{Mg}^{+2}$
and the standard hydrogen electrode as the cathode
$\mathrm{H}_{2} \rightarrow 2 \mathrm{H}^{+}$
Cell reaction is
$\mathrm{Mg} \mid \mathrm{Mg}^{2+}(\mathrm{aq} \cdot 1 \mathrm{M}) \| \mathrm{H}^{+}($aq. $1 \mathrm{M}) \mid \mathrm{H}_{2}(\mathrm{~g} .1 \mathrm{bar}) \cdot \mathrm{Pt}_{(\mathrm{s})}$
Then, the emf of the cell is measured and this measured emf is the standard electrode potential of the magnesium electrode.
$\mathrm{E}^{\circ}=\mathrm{E}_{\mathrm{c}}^{\mathrm{o}}-\mathrm{E}_{\mathrm{a}}^{\mathrm{o}}$
Here, $\mathrm{E}_{\mathrm{R}}^{0}$ for the standard hydrogen electrode is taken to be zero.
$\mathrm{E}^{\circ}=0-\mathrm{E}_{\mathrm{a}}^{\mathrm{o}}$
$=-E_{a}^{o}$
Concept insight:
The electrode potential of hydrogen is zero.
Question 2. Can you store copper sulphate solutions in a zinc pot? [2 Marks]
Solution. Zinc is more reactive than copper. Therefore, zinc can displace copper from its salt solution. If copper sulphate solution is stored in a zinc pot, then zinc will displace copper from the copper sulphate solution.
$\mathrm{Zn}+\mathrm{CuSO}_{4} \rightarrow \mathrm{ZnSO}_{4}+\mathrm{Cu}$
Hence, copper sulphate solution cannot be stored in a zinc pot.
Question 3. Consult the table of standard electrode potentials and suggest three substances that can oxidise ferrous ions under suitable conditions. [1 mark]
Solution. Substances that are stronger oxidizing agents than ferrous ions, that can oxidise the ferrous ions.
$\mathrm{Fe}^{2+} \rightarrow \mathrm{Fe}^{3+}+\mathrm{e}^{-1} ; \mathrm{E}^{0}=-0.77 \mathrm{~V}$
$\mathrm{F}_{2}(\mathrm{~g})+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{~F}^{-} \mathrm{E}^{\circ}=2.87$
$\mathrm{Co}^{3+}+\mathrm{e}^{-} \rightarrow \mathrm{Co}^{2+} \mathrm{E}^{0}=1.81$
$\mathrm{H}_{2} \mathrm{O}_{2}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{H}_{2} 0 \mathrm{E}^{\circ}=1.78$
$\mathrm{MnO}^{4-}+8 \mathrm{H}^{+}+5 \mathrm{e}^{-} \rightarrow \mathrm{Mn}^{2+}+4 \mathrm{H}_{2} 0 \mathrm{E}^{\circ}=1.51$
$\mathrm{Au}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Au}(\mathrm{s}) \mathrm{E}^{\circ}=1.40$
$\mathrm{Cl}_{2}(\mathrm{~g})+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Cl}^{-} \mathrm{E}^{0}=1.36$
$\mathrm{Cr}_{2} \mathrm{O}_{72}^{-}+14 \mathrm{H}^{+}+6 \mathrm{e}^{-} \rightarrow 2 \mathrm{Cr}^{3+}+7 \mathrm{H}_{2} 0 \mathrm{E}^{\circ}=1.33$
$\mathrm{O}_{2}(\mathrm{~g})+4 \mathrm{H}^{+}+4 \mathrm{e}^{-} \rightarrow 2 \mathrm{H}_{2} \mathrm{O} \mathrm{E}^{\circ}=1.23$
$\mathrm{MnO}_{2}(\mathrm{~s})+4 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Mn}^{2+}+2 \mathrm{H}_{2} \mathrm{O} \mathrm{E}^{\circ}=1.23$
$\mathrm{Br}_{2}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Br}^{-} \mathrm{E}^{\circ}=1.09$
$\mathrm{NO}^{3-}+4 \mathrm{H}^{+}+3 \mathrm{e}^{-} \rightarrow \mathrm{NO}(\mathrm{g})+2 \mathrm{H}_{2} \mathrm{O} \mathrm{E}^{\circ}=0.97$
This implies that the substances having higher reduction potentials than $+0.77 \mathrm{~V}$ can oxidise ferrous ions to ferric ions. Three substances that can do so are $\mathrm{MnO}^{-4}$, $\mathrm{Co}^{+3}, \mathrm{H}_{2} \mathrm{O}_{2}, \mathrm{Au}^{+3}, \mathrm{Cr}_{2} \mathrm{O}_{7}^{-2}, \mathrm{~F}_{2}, \mathrm{Cl}_{2}$ and $\mathrm{O}_{2} \mathrm{MnO}_{2}$
Concept insight:
The higher the reduction potential of the substance than Ferrous will oxidize ferrous.
Question 4. Calculate the potential of hydrogen electrode in contact with a solution whose pH is 10. [2 Marks]
Solution: Given: pH of solution is 10.
Take $\mathrm{T}=298 \mathrm{~K}, \mathrm{~F}=96500, \mathrm{R}=8.314$
For hydrogen electrode, $\mathrm{H}^{+}+\mathrm{e}^{-} \rightarrow \frac{1}{2} \mathrm{H}_{2}$,
Solution $\mathrm{pH}=10$
$\left[\mathrm{H}^{+}\right]=10^{-10} \mathrm{M}$
Now, using Nernest equation:
$\mathrm{E}_{\left(\mathrm{H}^{+} / \frac{1}{2} \mathrm{H}_{2}\right)}=\mathrm{E}_{\left(\mathrm{H}^{+} / \mathrm{H}_{2}\right)}^{0}-\frac{\mathrm{RT}}{\mathrm{nF}} \ln \frac{\mathrm{H}_{2}}{\left[\mathrm{H}^{+}\right]}$
$\therefore \mathrm{P}_{\mathrm{H}_{2}}=$ unity (1)
$=\mathrm{E}_{\left(\mathrm{H}^{+} / \mathrm{H}_{2}\right)}^{0}-\frac{0.0591}{1} \log \frac{1}{\left[\mathrm{H}^{+}\right]}$
$=0-\frac{0.0591}{1} \log \frac{1}{\left[10^{-10}\right]}$
$=-0.0591 \log 10^{10}$
$=-0.0591 \times 10 \mathrm{~V}$
$=-0.591 \mathrm{~V}$
Concept insight:
$\mathrm{pH}=-\log \left[\mathrm{H}^{+}\right]$
Nernest Equation is $\mathrm{E}_{\left(\mathrm{M}^{+1} / \mathrm{M}\right)}=\mathrm{E}_{\left(\mathrm{M}^{\mathrm{n}+} / \mathrm{M}\right)}^{0}-\frac{\mathrm{RT}}{\mathrm{nF}} \log \frac{[\mathrm{M}]}{\left[\mathrm{M}^{\mathrm{n}+}\right]}$
Where the concentration of solid M is taken as unity.
Question 5. Calculate the emf of the cell in which the following reaction takes place:
$\mathrm{Ni}(\mathrm{s})+2 \mathrm{Ag}^{+}(0.002 \mathrm{M}) \rightarrow \mathrm{Ni}^{2+}(0.160 \mathrm{M})$
$+2 \mathrm{Ag}(\mathrm{s})$ [2 Marks $]$
Given that $\mathrm{E}_{\text {(cell) }}^{0}=1.05 \mathrm{~V}$
Solution: Applying Nernst equation we have:
$\mathrm{E}_{(\text {cell })}=\mathrm{E}_{(\text {cell })}^{0}-\frac{0.0591}{\mathrm{n}} \log \frac{\left[\mathrm{Ni}^{2+}\right][\mathrm{Ag}]}{\left[\mathrm{Ag}^{+}\right]^{2}[\mathrm{Ni}]}$
Active mass of solid is taken to be unity so $\mathrm{Ni}(\mathrm{s})=\mathrm{Ag}(\mathrm{s})=1$
$E_{(\text {cell })}=E_{(\text {cell })}^{0}-\frac{0.0591}{n} \log \frac{\left[\mathrm{Ni}^{2+}\right]}{\left[\mathrm{Ag}^{+}\right]^{2}}$
$=1.05-\frac{0.0591}{2} \log \frac{(0.160)}{(0.002)^{2}}$
$=1.05-0.02955 \log \frac{0.16}{0.000004}$
$=1.05-0.02955 \log \left(4 \times 10^{4}\right)$
$=1.05-0.02955\left(\log 10^{4}+\log 4\right)$
$=1.05-0.02955\left(4 \log 10+\log 2^{2}\right)$
$=1.05-0.02955(4 \log 10+2 \log 2)$
$=1.05-0.02955(4+0.6021)$
$\therefore \log 2=0.3010$ and $\log 10=1$
$=0.914 \mathrm{~V}$
Question 6. The cell in which the following reactions occurs: [3 Marks]
$2 \mathrm{Fe}^{3+}(\mathrm{aq})+2 \mathrm{I}^{-}(\mathrm{aq}) \rightarrow 2 \mathrm{Fe}^{2+}(\mathrm{aq})+\mathrm{I}_{2}(\mathrm{~s})$
Has $\mathrm{E}_{\text {cell }}^{0}=0.236 \mathrm{~V}$ at $298 \mathrm{~K}$
Calculate the standard Gibbs energy and the equilibrium constant of the cell reaction. [2 Marks]
Solution: Given $\mathrm{E}_{\text {cell }}^{0}=0.236 \mathrm{~V}$
$\mathrm{T}=298 \mathrm{~K}$
$2 \mathrm{Fe}^{3+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Fe}^{2+}(\mathrm{aq})$
$2 \mathrm{I}^{-}(\mathrm{aq}) \rightarrow \mathrm{I}_{2}(\mathrm{~s})+2 \mathrm{e}^{-}$
Here, $\mathrm{n}=2$ and $\mathrm{F}=96500$
We know that:
$\Delta_{\mathrm{r}} \mathrm{G}^{0}=-\mathrm{nFE}_{\text {cell }}^{0}$
$=-2 \times 96500 \times 0.236$
$=-45548 \mathrm{Jmol}^{-1}$
$=-45.54 \mathrm{k} \mathrm{J} \mathrm{mol}^{-1}=-45.54 \mathrm{X} 10^{3} \mathrm{~J} \mathrm{~mol}^{-1}$
Again, $\Delta_{\mathrm{r}} \mathrm{G}=\Delta_{\mathrm{r}} \mathrm{G}^{0}+2.303 \mathrm{RT} \log \mathrm{Q}$
at equilibrium $\therefore \Delta_{\mathrm{r}} \mathrm{G}=0$ and $\mathrm{Q}=\mathrm{K}_{\mathrm{c}}$
$\Delta_{\mathrm{r}} \mathrm{G}^{0}=-2.303 \mathrm{RT} \log \mathrm{K}_{\mathrm{c}}$
$\Rightarrow \log \mathrm{K}_{\mathrm{c}}=-\frac{\Delta_{\mathrm{r}} \mathrm{G}^{\circ}}{2.303 \mathrm{RT}}$
$=-\frac{\left(-45.54 \times 10^{3}\right)}{2.303 \times 8.314 \times 298}$
$=7.981$
$\therefore \mathrm{K}_{\mathrm{c}}=$ Antilog $(7.981)$
$=9.57 \times 10^{7}$
Question 7. Why does the conductivity of a solution decrease with dilution? [1 mark]
Solution. The conductivity of a solution is the conductance of ions presenting a unit volume of the solution. The number of ions (responsible for carrying current) decreases when the solution is diluted. As a result, the conductivity of a solution decreases with dilution.
Question 8. Suggest a way to determine the $\Lambda_{\mathrm{m}}^{0}$ value of water. [2 Marks]
Solution. According to kohlrausch’s law at infinite dilution each ion makes a definite contribution towards equivalent conductance of electrolyte irrespective of the nature of ion with which it is associated and the value of equivalent conductance at infinite dilution for any electrolyte is the sum of contributions from its constituent ions
Applying Kohlrausch's law of independent migration of ions, the $\wedge_{\mathrm{m}}^{0}$ value of water can be determined as follows:
$\Lambda_{\mathrm{m}\left(\mathrm{H}_{2} \mathrm{O}\right)}^{0}=\lambda_{\mathrm{H}^{-}}^{0}+\lambda_{\mathrm{OH}^{-}}^{0}$
$=\left(\lambda_{\mathrm{H}^{-}}^{0}+\lambda_{\mathrm{CI}^{-}}^{0}\right)+\left(\lambda_{\mathrm{Na}^{+}}^{0}+\lambda_{\mathrm{OH}^{-}}^{0}\right)-\left(\lambda_{\mathrm{Na}^{+}}^{0}+\lambda_{\mathrm{Cl}^{-}}^{0}\right)$
$=\Lambda_{\mathrm{m}(\mathrm{HCl})}^{0}+\Lambda_{\mathrm{m}(\mathrm{NaOH})}^{0}-\Lambda_{\mathrm{m}(\mathrm{NaCl})}^{0}$
Hence, by knowing the $\Lambda_{\mathrm{m}}^{0}$ values of $\mathrm{HCl}, \mathrm{NaOH}$ and $\mathrm{NaCl}$, the $\wedge_{\mathrm{m}}^{0}$ value of water can be determined.
Concept insight:
Limiting molar conductivity of an electrolyte can be represented as the sum of the individual contributions of anion and cation of the electrolyte.
Question 9. The molar conductivity of $0.025 \mathrm{~mol} \mathrm{~L}^{-1}$ methanoic acid is $46.1 \mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}$.
Calculate its degree of dissociation and dissociation constant. Given
$\lambda^{\circ}\left(\mathrm{H}^{+}\right)=349.6 \mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}$ and $\lambda^{\circ}\left(\mathrm{HCOO}^{-}\right)$
$=54.6 \mathrm{~S} \mathrm{~cm}^{2}$ mol. [2 Marks]
Solution: Given:
$\mathrm{C}=0.025 \mathrm{~mol} \mathrm{~L}^{-1}$
$\wedge_{\mathrm{m}}=46.1 \mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}$
$\lambda^{0}\left(\mathrm{H}^{+}\right)=349.6 \mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}$
$\lambda^{0}\left(\mathrm{HCOO}^{-}\right)=54.6 \mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}$
$\wedge_{\mathrm{m}}^{0}(\mathrm{HCOOH})=\lambda^{0}\left(\mathrm{H}^{+}\right)+\lambda^{0}\left(\mathrm{HCOO}^{-}\right)$
$=349.6+54.6$
$=404.2 \mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}$
Now, degree of dissociation:
$\alpha=\frac{\Lambda_{\mathrm{m}}(\mathrm{HCOOH})}{\Lambda_{\mathrm{m}}^{0}(\mathrm{HCOOH})}$
$=\frac{46.1}{404.2}$
$=0.114$ (approximately)
Thus, dissociation constant:
$\mathrm{K}=\frac{\mathrm{c} \propto^{2}}{(1-\propto)}$
$=\frac{\left(0.025 \mathrm{~mol} \mathrm{~L}^{-1}\right)(0.114)^{2}}{(1-0.114)}=\frac{\left(0.025 \mathrm{~mol} \mathrm{~L}^{-1}\right)(0.114)^{2}}{(0.886)}$
$=3.67 \times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1}$
Concept insight:
This is very important from examination point.
Degree of dissociation $\propto$ is the ratio of the molar conductivity at the concentration c to limiting molar conductivity.
Question 10. If a current of $0.5$ ampere flows through a metallic wire for 2 hours, then how many electrons would flow through the wire? [2 Marks]
Solution. Given:
current $(\mathrm{I})=0.5$ ampere
time $(\mathrm{t})=2$ hours $=2 \times 60 \times 60 \mathrm{~s}=7200 \mathrm{~s}$
Find charge $(\mathrm{Q})=$ ?
Formula: charge (Q) $=\operatorname{current}(\mathrm{I}) \times \operatorname{time}(\mathrm{t})$ in second
$=0.5 \mathrm{~A} \times 7200 \mathrm{~s}$
$=3600 \mathrm{C}$
We know that $1 \mathrm{~F}=96500 \mathrm{C}$ charge $=6.023 \times 10^{23}$ number of electrons.
Then, $3600 \mathrm{C}=\frac{6.023 \times 10^{23} \times 3600}{96500}$ number of electrons
$=2.28 \times 10^{22}$ number of electrons
Hence, $2.28 \times 10^{22}$ number of electrons will flow through the wire.
Concept insight:
Question 11. Suggest a list of metals that are extracted electrolytically. [1 Marks]
Solution. Products of electrolysis depend on the nature of material being electrolysed and the type of electrodes being used. If the electrode is inert (e.g., platinum or gold), it does not participate in the chemical reaction and acts only as source or sink for electrons. On the other hand, if the electrode is reactive, it participates in the electrode reaction. Thus, the products of electrolysis may be different for reactive and inert Electrodes. The products of electrolysis depend on the different oxidizing and reducing species present in the electrolytic cell and their standard electrode potentials. Moreover, some of the electrochemical processes although feasible, are so slow kinetically that at lower voltages these do not seem to take place and extra potential (called overpotential) has to be applied, which makes such process more difficult to occur. Metals that are on the top of the reactivity series such as sodium, potassium, calcium, lithium, magnesium, aluminium are extracted electrolytically.
Question 12. What is the quantity of electricity in coulombs needed to reduce $1 \mathrm{~mol}$ of $\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}$ ?
Consider the reaction: $\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}+14 \mathrm{H}^{+}+6 \mathrm{e}^{-} \rightarrow 2 \mathrm{Cr}^{3+}+7 \mathrm{H}_{2}$ 0. [2 Marks $]$
Solution: Given:
mol of $\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}=1$
The given reaction is as follows:
$\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}+14 \mathrm{H}^{+}+6 \mathrm{e}^{-} \rightarrow 2 \mathrm{Cr}^{3+}+7 \mathrm{H}_{2} \mathrm{O}$
We know: Therefore, to reduce 1 mole of $\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}$ need 6 mole of electron,
Charge on one mole of electron = 1F
$1 \mathrm{~F}=96500$
the required quantity of electricity will be:
= 6 F (because 6 mole electrons are using there)
$=6 \times 96500 \mathrm{C}$
$=579000 \mathrm{C}$
Question 13. Write the chemistry of recharging the lead storage battery, highlighting all the materials that are involved during recharging. [3 Marks]
Solution. A lead storage battery consists of a lead anode, a grid of lead packed with lead oxide $\left(\mathrm{Pb} \mathrm{O}_{2}\right)$ as the cathode and a $38 \%$ solution of sulphuric acid $\left(\mathrm{H}_{2} \mathrm{SO}_{4}\right)$ as an electrolyte.
When the battery is in use, the following cell reactions take place:
At anode: $\mathrm{Pb}(\mathrm{s})+\mathrm{SO}_{4}^{2-}(\mathrm{aq}) \rightarrow \mathrm{PbSO}_{4}(\mathrm{~s})+2 \mathrm{e}^{-}$
At cathode: $\mathrm{Pb}(\mathrm{s})+\mathrm{SO}_{4}^{2-}(\mathrm{aq})+4 \mathrm{H}^{+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{PbSO}_{4}(\mathrm{~s})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{l})$
The overall cell reaction is given by,
$\mathrm{Pb}(\mathrm{s})+\mathrm{PbO}_{2}(\mathrm{~s})+2 \mathrm{H}_{2} \mathrm{SO}_{4}(\mathrm{aq}) \rightarrow 2 \mathrm{PbSO}_{4}(\mathrm{~s})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{l})$
When a battery is charged, the reverse of all these reactions takes place.
At anode: $\mathrm{PbSO}_{4}(\mathrm{~s})+2 \mathrm{e}^{-} \rightarrow \mathrm{Pb}(\mathrm{s})+\mathrm{SO}_{4}^{2-}(\mathrm{aq})$
At cathode: $\mathrm{PbSO}_{4}(\mathrm{~s})+2 \mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{PbO}_{2}(\mathrm{~s})+\mathrm{SO}_{4}^{2-}(\mathrm{aq})$
Overall Reaction $\mathrm{PbSO}_{4}(\mathrm{~s})+2 \mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{Pb}(\mathrm{s})+\mathrm{PbO}_{2}(\mathrm{~s})$
Hence, on charging, $\mathrm{PbSO}_{4}(\mathrm{~s})$ present at the anode and cathode is converted into $\mathrm{Pb}(\mathrm{s})$ and $\mathrm{PbO}_{2}$ (s) respectively.
Question 14. Suggest two materials other than hydrogen that can be used as fuels in fuel cells. [1 mark]
Solution. Galvanic cells that are designed to convert, the energy of combustion of fuels, hydrogen, methane, methanol are directly into electrical energy are called fuel cells.
Concept insight:
Cells that are designed to convert the energy of combustion of fuels directly into electrical energy are called fuel cells.
Question 15. Explain how rusting of iron is envisage as setting up of an electrochemical cell. [2 Marks]
Solution. In the process of corrosion, due to the presence of air and moisture, oxidation takes place at a particular spot of an object made of iron. That spot behaves as the anode.
The reaction at the anode is given by,
$\mathrm{Fe}(\mathrm{s}) \rightarrow \mathrm{Fe}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-}$(oxidation)
$\mathrm{E}(\mathrm{Fe}+2 / \mathrm{Fe})=-0.44 \mathrm{~V}$
Electrons released at the anodic spot move through the metallic object and go to another spot of the object.
There, in the presence of $\mathrm{H}^{+}$ions, the electrons reduce oxygen. This spot behaves as the cathode. These $\mathrm{H}^{+}$ions come either from $\mathrm{H}_{2} \mathrm{CO}_{3}$, which are formed due to the dissolution of carbon dioxide from air into water or from the dissolution of other acidic oxides from the atmosphere in water.
The reaction corresponding at the cathode is given by,
$\mathrm{O}_{2}(\mathrm{~g})+4 \mathrm{H}^{+}(\mathrm{aq})+4 \mathrm{e}^{-} \rightarrow 2 \mathrm{H}_{2} \mathrm{O}$ (l)(reduction)
The overall reaction is:
$2 \mathrm{Fe}(\mathrm{s})+\mathrm{O}_{2}(\mathrm{~g})+4 \mathrm{H}^{+}(\mathrm{aq}) \rightarrow 2 \mathrm{Fe}^{2+}(\mathrm{aq})+2 \mathrm{H}_{2} \mathrm{O}$ (l)
Also, ferrous ions $\left(\mathrm{Fe}^{+2}\right)$ are further oxidized by atmospheric oxygen to ferric ions $\left(\mathrm{Fe}^{+3}\right)$. These ferric ions combine with moisture, present in the surroundings, to form hydrated ferric oxide $\left(\mathrm{Fe}_{2} \mathrm{O}_{3}, \mathrm{xH}_{2} \mathrm{O}\right)$ i.e., rust.
Hence, the rusting of iron is envisaged as the setting up of an electrochemical cell.
Concept insight:
Corrosion of metals is essentially an electrochemical phenomenon.
(BOC)
Question 1. Arrange the following metals in the order in which they displace each other from the solution of their salts.
Al, Cu, Fe, Mg and Zn. [2 Marks]
Solution. The following is the order in which the given metals displace each other from the solution of their salts. metal which is placed above the electrochemical series displaced the metal which is lower in series .
A metal of stronger reducing power (placed above the electrochemical series) displaces another metal of weaker reducing power (placed below the electrochemical series) from its solution of salt.
The order of the increasing reducing power of the given metals is $\mathrm{Cu}<\mathrm{Fe}<\mathrm{Zn}<$ $\mathrm{Al}<\mathrm{Mg}$. Hence, we can say that $\mathrm{Mg}$ can displace Al from its salt solution, but Al cannot displace $\mathrm{Mg}$. Thus, the order in which the given metals displace each other from the solution of their salts is given below: $\mathrm{Mg}>\mathrm{Al}>\mathrm{Zn}>\mathrm{Fe}>\mathrm{Cu}$
Concept insight:
The lower the value of reduction potential easily it displaces from the solution.
Question 2. Given the standard electrode potentials,
$\mathrm{K}+/ \mathrm{K}=-2.93 \mathrm{~V}$
$\mathrm{Ag}^{+} / \mathrm{Ag}=0.80 \mathrm{~V}$
$\mathrm{Hg}^{2+} / \mathrm{Hg}=0.79 \mathrm{~V}$
$\mathrm{Mg}^{2+} / \mathrm{Mg}=-2.37 \mathrm{~V}$
$\mathrm{Cr}^{3+} / \mathrm{Cr}=-0.74 \mathrm{~V}$
Arrange these metals in their increasing order of reducing power. [2 Marks]
Solution. The given standard electrode potentials increase in the order of $\mathrm{K}^{+} / \mathrm{K}<$ $\mathrm{Mg}^{2+} / \mathrm{Mg}<\mathrm{Cr}^{3+} / \mathrm{Cr}<\mathrm{Hg}^{2+} / \mathrm{Hg}<\mathrm{Ag}^{+} / \mathrm{Ag}$.
The lower the reduction potential, the higher is the reducing power.
Hence, the reducing power of the given metals increases in the following order:
$\mathrm{Ag}<\mathrm{Hg}<\mathrm{Cr}<\mathrm{Mg}<\mathrm{K}$
Concept insight:
Lower the value of reduction potential, Higher is the reducing power.
Question 3. Depict the galvanic cell in which the reaction $\mathrm{Zn}(\mathrm{s})+2 \mathrm{Ag}^{+}(\mathrm{aq}) \rightarrow \mathrm{Zn}^{2+}(\mathrm{aq})+$
$2 \mathrm{Ag}(\mathrm{s})$ takes place. Further show:
(i) Which of the electrode is negatively charged?
(ii) The carriers of the current in the cell.
(iii) Individual reaction at each electrode. [3 Marks]
Solution: The galvanic cell in which the given reaction takes place is depicted as:
$\mathrm{Zn}(\mathrm{s})\left|\mathrm{Zn}^{2+}(\mathrm{aq})\right| \mathrm{Ag}^{+}(\mathrm{aq}) \mid \mathrm{Ag}(\mathrm{s})$
(i) Zn electrode (anode) is negatively charged
(ii) Ions are carriers of current in the cell (internal circuit) by the slat bridge and in the external circuit (opposite electron flow), current will flow from silver to zinc due to presence of electrons
(iii) The oxidation reaction taking place at the anode is given by,
$\mathrm{Zn}(\mathrm{s}) \rightarrow \mathrm{Zn}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-}$
The reduction reaction taking place at the cathode is given by,
$\mathrm{Ag}^{+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{Ag}(\mathrm{s})$
Concept insight:
Anode is negatively charged as the oxidation takes place.
In the cell the ions are carriers as there is presence of salt bridge whereas in the external circuit electrons are the carriers.
Question 4. Calculate the standard cell potentials of galvanic cells in which the following reactions take place:
(i) $2 \mathrm{Cr}(\mathrm{s})+3 \mathrm{Cd}^{2+}(\mathrm{aq}) \rightarrow 2 \mathrm{Cr}^{3+}(\mathrm{aq})+3 \mathrm{Cd}$
(ii) $\mathrm{Fe}^{2+}(\mathrm{aq})+\mathrm{Ag}^{+}(\mathrm{aq}) \rightarrow \mathrm{Fe}^{3+}(\mathrm{aq})+\mathrm{Ag}(\mathrm{s})$
Calculate the $\Delta_{\mathrm{r}} \mathrm{G}^{0}$ and equilibrium constant of the reactions. [4 Marks]
Solution. (i) $2 \mathrm{Cr}(\mathrm{s})+3 \mathrm{Cd}^{2+}(\mathrm{aq}) \rightarrow 2 \mathrm{Cr}^{3+}(\mathrm{aq})+3 \mathrm{Cd}$
Anode: $\operatorname{Cr}(s) \rightarrow \operatorname{Cr}^{3+}(a q)+3 e^{-} E_{C r^{3+} / C r}^{0}=0.74 V$
Cathode: $\mathrm{Cd}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Cd}(\mathrm{s}) \mathrm{E}_{\mathrm{Cd}^{2+} / \mathrm{Cd}}^{0}=-0.40 \mathrm{~V}$
The galvanic cell of the given reaction is depicted as:
$\mathrm{Cr}(\mathrm{s})\left|\mathrm{Cr}^{3+}(\mathrm{aq}) \| \mathrm{Cd}^{2+}(\mathrm{aq})\right| \mathrm{Cd}(\mathrm{s})$
$\left[\mathrm{Cr} \rightarrow \mathrm{Cr}^{+3}+3 \mathrm{e}^{-}\right] \times 2$
$\left[2 \mathrm{e}^{-}+\mathrm{Cd}^{+2} \rightarrow \mathrm{Cd}\right] \times 3$
Overall reaction : $2 \mathrm{Cr}(\mathrm{s})+3 \mathrm{Cd}^{2+}(\mathrm{aq}) \rightarrow 2 \mathrm{Cr}^{3+}(\mathrm{aq})+3 \mathrm{Cd}$
Hence
Now, the standard cell potential is
$\mathrm{E}_{\text {cell }}^{0}=\mathrm{E}_{\text {cathode }}^{0}-\mathrm{E}_{\text {anode }}^{0}$
$\mathrm{E}_{\text {cell }}^{0}=-0.40-(-0.74)$
$\mathrm{E}_{\mathrm{cell}}^{0}=+0.34 \mathrm{~V}$
$\Delta_{\mathrm{r}} \mathrm{G}^{0}=-\mathrm{nFE}_{\mathrm{cell}}^{0}$
In the given equation, $2 \mathrm{Cr}(\mathrm{s})+3 \mathrm{Cd}^{2+}(\mathrm{aq}) \rightarrow 2 \mathrm{Cr}^{3+}(\mathrm{aq})+3 \mathrm{Cd}$
$\mathrm{n}=6$
$\mathrm{F}=96500 \mathrm{C} \mathrm{mol}^{-1}$
$\mathrm{E}_{\text {cell }}^{0}=+0.34$ Then
$\Delta_{\mathrm{r}} G^{0}=-\mathrm{nFE}_{\text {cell }}^{0}=-6 \times 96500 \mathrm{Cmol}^{-1} \times 0.34 \mathrm{~V}$
$=-196860 \mathrm{CV} \mathrm{mol}^{-1}$
$=-196860 \mathrm{~mol}^{-1}$
$=-196.860 \mathrm{~kJ} \mathrm{~mol}^{-1}$
Again,
$\Delta_{\mathrm{r}} \mathrm{G}=\Delta_{\mathrm{r}} \mathrm{G}^{0}+\mathrm{RT} \ln \mathrm{Q}$
At equilibrium
$\Delta_{\mathrm{r}} \mathrm{G}=0$ and $\mathrm{Q}=\mathrm{K}$
$\Delta_{\mathrm{r}} \mathrm{G}^{0}=-\mathrm{RT} \ln \mathrm{K}$
$\Rightarrow \Delta_{\mathrm{r}} \mathrm{G}^{0}=-\mathrm{RT} \ln \mathrm{K}$
$\Rightarrow \Delta_{\mathrm{r}} \mathrm{G}^{0}=-2.303 \mathrm{RT} \log \mathrm{K}$
$\Rightarrow \ln \mathrm{K}=-\frac{\Delta_{\mathrm{r}} \mathrm{G}}{2.303 \mathrm{RT}}$
$\Rightarrow \ln \mathrm{K}=\frac{-196.86 \times 10^{3}}{2.303 \times 8.314 \times 298}$
$\Rightarrow \ln \mathrm{K}=34.47$
$\Rightarrow$ Anti log of $34.7=3.193 \times 10^{34}$
$\mathrm{k}_{\mathrm{c}}=3.192 \times 10^{34}$
(ii) $\mathrm{Fe}^{2+}(\mathrm{aq})+\mathrm{Ag}^{+}(\mathrm{aq}) \rightarrow \mathrm{Fe}^{3+}(\mathrm{aq})+\mathrm{Ag}(\mathrm{s})$
The galvanic cell of the given reaction is represented as
$\mathrm{Fe}^{2+}(\mathrm{aq})\left|\mathrm{Fe}^{3+}(\mathrm{aq})\right|\left|\mathrm{Ag}^{+}\right| \mathrm{Ag}(\mathrm{s})$
Anode $: \mathrm{Fe}^{2+}(\mathrm{aq}) \rightarrow \mathrm{Fe}^{3+}(\mathrm{aq})+\mathrm{e}^{-} \mathrm{E}_{\mathrm{cell}}^{0}=0.77$
Cathode : $\mathrm{Ag}^{+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{Ag}(\mathrm{s}) \quad \mathrm{E}_{\mathrm{cell}}^{0}=0.80$

check here to get all values
$\mathrm{E}_{\mathrm{cell}}^{0}=0.80-0.77$
$\mathrm{E}_{\text {cell }}^{0}=+0.03 \mathrm{~V}$
In balanced reaction there are 1 electron are transferring so that n = 1
Faraday constant, $\mathrm{F}=96500 \mathrm{C} \mathrm{mol}^{-1}$
$\mathrm{E}_{\mathrm{cell}}^{\mathrm{o}}=+0.03 \mathrm{~V}$
Use formula
$\Delta_{\mathrm{r}} \mathrm{G}^{0}=-\mathrm{nFE}_{\text {cell }}^{\mathrm{o}}$
Plug the value we get
Then, $=-1 \times 96500 \mathrm{Cmol}^{-1} \times 0.03 \mathrm{~V}$
$=-2895 \mathrm{CV} \mathrm{mol}^{-1}$
$=-2895 \mathrm{Jmol}^{-1}$
$=-2.895 \mathrm{~kJ} \mathrm{~mol}^{-1}$
Again,
$\Delta_{\mathrm{r}} \mathrm{G}=\Delta_{\mathrm{r}} \mathrm{G}^{0}+\mathrm{RT} \ln \mathrm{Q}$
at equilibrium $Q=K$ and $\Delta_{\mathrm{r}} \mathrm{G}=0$
$\Delta_{\mathrm{r}} \mathrm{G}^{0}=-\mathrm{RT} \ln \mathrm{K}$
$\Rightarrow \Delta_{\mathrm{r}} G^{0}=-2.303 \mathrm{RT} \ln \mathrm{K}$
$\Rightarrow \Delta_{\mathrm{r}} \mathrm{G}^{0}=-2.303 \mathrm{RT} \ln \mathrm{K}$
$\Rightarrow \log \mathrm{K}=-\frac{\Delta_{\mathrm{r}} \mathrm{G}}{2.303 \mathrm{RT}}$
$\Rightarrow \log \mathrm{K}=-\frac{-2895}{2.303 \times 8.314 \times 298}$
$\Rightarrow \log \mathrm{K}=3.22$
Take antilog both side we get
$\therefore$ Anti log of $3.22=1659.5869074375614$
$\mathrm{K}=1659.5869074375614$
Question 5. Write the Nernst equation and emf of the following cells at $298 \mathrm{~K}$ :
(i) $\mathrm{Mg}(\mathrm{s})\left|\mathrm{Mg}^{2+}(0.001 \mathrm{M}) \| \mathrm{Cu}^{2+}(0.0001 \mathrm{M})\right| \mathrm{Cu}(\mathrm{s})$
(ii) $\mathrm{Fe}(\mathrm{s})\left|\mathrm{Fe}^{2+}(0.001 \mathrm{M}) \| \mathrm{H}^{+}(1 \mathrm{M})\right| \mathrm{H}_{2}(\mathrm{~g})(1 \mathrm{bar}) \mid \mathrm{Pt}(\mathrm{s})$
(iii) $\mathrm{Sn}(\mathrm{s})\left|\mathrm{Sn}^{2+}(0.050 \mathrm{M}) \| \mathrm{H}^{+}(0.020 \mathrm{M})\right| \mathrm{H}_{2}(\mathrm{~g})(1 \mathrm{bar}) \mid \mathrm{Pt}(\mathrm{s})$
(iv) $\mathrm{Pt}(\mathrm{s})\left|\mathrm{Br}_{2}(\mathrm{l})\right| \mathrm{Br}^{-}(0.010 \mathrm{M}) \| \mathrm{H}^{+}(0.030 \mathrm{M})\left|\mathrm{H}_{2}(\mathrm{~g})(1 \mathrm{bar})\right| \mathrm{Pt}(\mathrm{s})[4$ Marks $]$
Solution: (i) For the given reaction, $\mathrm{Mg}(\mathrm{s})\left|\mathrm{Mg}^{2+}(0.001 \mathrm{M}) \| \mathrm{Cu}^{2+}(0.0001 \mathrm{M})\right| \mathrm{Cu}(\mathrm{s})$
Cathode $: \mathrm{Mg}(\mathrm{s}) \rightarrow \mathrm{Mg}^{2+}(\mathrm{aq})$
Anode:Cu $^{2+}($ aq $) \rightarrow \mathrm{Cu}(\mathrm{s})$
the Nernst equation can be given as:
$\Delta_{\mathrm{r}} \mathrm{G}=\Delta_{\mathrm{r}} \mathrm{G}^{0}+\mathrm{RT} \ln \mathrm{Q}$
$\therefore \Delta_{\mathrm{r}} \mathrm{G}=\mathrm{nFE}, \Delta_{\mathrm{r}} \mathrm{G}^{0}=-\mathrm{nFE}_{\mathrm{cell}}^{0}$
$-n F \Delta_{r} E_{c e l l}=-n F \Delta_{r} E_{c e l l}^{0}+R T \ln Q$
$Q=\frac{\left[M g^{2+}\right][C u(s)]}{\left[C u^{2+}\right][M g(s)]}$ and active mass of pure solid is taken to be unity so
$[\mathrm{Cu}(\mathrm{s})]=[\mathrm{Mg}(\mathrm{s})]=1$
Hence $Q=\frac{\left[\mathrm{Mg}^{2+}\right]}{\left[\mathrm{Cu}^{2+}\right]}$
$\mathrm{E}_{\mathrm{cell}}=\mathrm{E}_{\mathrm{cell}}^{0}-\frac{0.0591}{\mathrm{n}} \log \frac{\left[\mathrm{Mg}^{2+}\right]}{\left[\mathrm{Cu}^{2+}\right]}$
$=\{0.34-(-2.36)\}-\frac{0.0591}{2} \log \frac{0.001}{0.0001}$
$=2.7-\frac{0.0591}{2} \log 10$
$=2.7-0.02955$
$=2.67 \mathrm{~V}$ (approximately)
(ii) For the given reaction, $\mathrm{Fe}(\mathrm{s})\left|\mathrm{Fe}^{2+}(0.001 \mathrm{M}) \| \mathrm{H}^{+}(1 \mathrm{M})\right| \mathrm{H}_{2}(\mathrm{~g})(1 \mathrm{bar}) \mid \mathrm{Pt}(\mathrm{s})$
At anode $: \mathrm{Fe}(\mathrm{s}) \rightarrow \mathrm{Fe}^{+2}(\mathrm{aq})+2 \mathrm{e}^{-} \mathrm{E}^{0}=-0.44$
At cathode : $2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{H}_{2} \mathrm{E}^{0}=0$
the Nernst equation can be given as:
$\Delta_{\mathrm{r}} \mathrm{G}=\Delta_{\mathrm{r}} \mathrm{G}^{0}+\mathrm{RT} \ln \mathrm{Q}$
$\therefore \Delta_{\mathrm{r}} \mathrm{G}=\mathrm{nFE}, \Delta_{\mathrm{r}} \mathrm{G}^{0}=-\mathrm{nFE}_{\text {cell }}^{0}$
$-n F \Delta_{r} E_{c e l l}=-n F \Delta_{r} E_{c e \|}^{0}+R T \ln Q$
$Q=\frac{\left[\mathrm{Fe}^{+2}\right]\left[\mathrm{H}_{2}(\mathrm{~g})\right]}{\left[\mathrm{H}^{+}\right]^{2}[\mathrm{Fe}(\mathrm{s})]}$
$Q=\frac{\left[M g^{2+}\right][C u(s)]}{\left[C u^{2+}\right][M g(s)]}$ and active mass of pure solid is taken to be unity and
pressure of $\mathrm{H}_{2}$ gas is 1 atm.so $[\mathrm{Fe}(\mathrm{s})]=\left[\mathrm{H}_{2}(\mathrm{~g})\right]=1$
Hence $Q=\frac{[F e]}{\left[H^{+}\right]^{2}}$
$E_{\text {cell }}=E_{\text {cell }}^{0}-\frac{0.0591}{n} \log \frac{\left[\mathrm{Fe}^{2+}\right]}{\left[\mathrm{H}^{+}\right]^{2}}$
$=\{0-(-0.44)\}-\frac{0.0591}{2} \log \frac{0.001}{1^{2}}$
$=0.44-0.02955(-3)$
$=0.52865 \mathrm{~V}$
$=0.53 \mathrm{~V}$ (approximately)
(iii) For the given
reaction, $\mathrm{Sn}(\mathrm{s})\left|\mathrm{Sn}^{2+}(0.050 \mathrm{M}) \| \mathrm{H}^{+}(0.020 \mathrm{M})\right| \mathrm{H}_{2}(\mathrm{~g})(1 \mathrm{bar}) \mid \mathrm{Pt}(\mathrm{s})$ the
At anode: $\mathrm{Sn}(\mathrm{s}) \rightarrow \mathrm{Sn}^{+2}+2 \mathrm{e}^{-}$
At cathode: $2 \mathrm{H}^{+} \rightarrow \mathrm{H}_{2}$ ( 1 bar $)$
Nernst equation can be given as:
$\Delta_{\mathrm{r}} \mathrm{G}=\Delta_{\mathrm{r}} \mathrm{G}^{0}+\mathrm{RT} \ln \mathrm{Q}$
$\therefore \Delta_{\mathrm{r}} \mathrm{G}=-\mathrm{nFE}, \Delta_{\mathrm{r}} \mathrm{G}^{0}=-\mathrm{nFE}_{\text {cell }}^{0}$
$-\mathrm{nF} \Delta_{\mathrm{r}} \mathrm{E}_{\text {cell }}=-\mathrm{nF} \Delta_{\mathrm{r}} \mathrm{E}_{\text {cell }}^{0}+\mathrm{RT} \ln Q$
$\mathrm{Q}=\frac{\left[\mathrm{Sn}^{2+}\right]\left[\mathrm{H}_{2}(\mathrm{~g})\right]}{\left[\left[\mathrm{H}^{+}(\mathrm{aq})\right]^{2}\right][\mathrm{Sn}(\mathrm{s})]}$ and active mass of pure solid is taken to be unity so
$[\mathrm{Cu}(\mathrm{s})]=[\mathrm{Mg}(\mathrm{s})]=1$
Hence $Q=\frac{\left[\mathrm{Sn}^{2+}\right]}{\left[\mathrm{H}^{+}\right]^{2}}$
$\mathrm{E}_{\text {cell }}=\mathrm{E}_{\text {cell }}^{0}-\frac{0.0591}{\mathrm{n}} \log \frac{\left[\mathrm{Sn}^{2+}\right]}{\left[\mathrm{H}^{+}\right]^{2}}$
$=\{0-(-0.14)\}-\frac{0.0591}{2} \log \frac{0.050}{(0.020)^{2}}$
$=0.14-0.0295 \times \log 125$
$=0.14-0.062$
$=0.078 \mathrm{~V}$
$=0.08 \mathrm{~V}$ (approximately)
(iii) For the $\quad$ given $\quad$ reaction, $\operatorname{Pt}(\mathrm{s})\left|\mathrm{Br}_{2}(\mathrm{l})\right| \mathrm{Br}^{-}(0.010 \mathrm{M}) \| \mathrm{H}^{+}(0.030 \mathrm{M}) \mid \mathrm{H}_{2}(\mathrm{~g})(1$ bar $) \mid \mathrm{Pt}(\mathrm{s})$
at anode $\rightarrow 2 \mathrm{Br}^{-} \rightarrow \mathrm{Br}_{2}+2 \mathrm{e}^{-} \quad($ n factor $=2)$
Cathode $=2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{H}_{2}(\mathrm{~g}) \quad(\mathrm{n}$ factor $=2)$
Overall reaction : $2 \mathrm{Br}^{-}+2 \mathrm{H}^{+} \rightarrow \mathrm{Br}_{2}+\mathrm{H}_{2}$
$\mathrm{Q}=\frac{\left[\mathrm{Br}_{2}(\mathrm{l})\right]\left[\mathrm{H}_{2}(\mathrm{~g})\right]}{ \left.\left[\mathrm{IBr}^{-}(\mathrm{aq})\right]^{2}\right]\left[\left[\mathrm{H}^{+}(\mathrm{aq})\right]^{2}\right]}$ and active mass of pure solid and liquid is taken
to be unity so $\left[\mathrm{Br}_{2}(\mathrm{l})\right]=1$ and pressure of $\mathrm{H}_{2}=1 \mathrm{atmp}$
Hence $Q=\frac{1}{\left[\left[\mathrm{Br}^{-}(\mathrm{aq})\right]^{2}\right]\left[\left[\mathrm{H}^{+}(\mathrm{aq})\right]^{2}\right]}$
Hence $Q=\frac{1}{\left[\left[B r^{-}(a q)\right]^{2}\right]\left[\left[\mathrm{H}^{+}(a q)\right]^{2}\right]}$
the Nernst equation can be given as:
$\Delta_{\mathrm{r}} \mathrm{G}=\Delta_{\mathrm{r}} \mathrm{G}^{0}+\mathrm{RT} \ln \mathrm{Q}$
$\therefore \Delta_{\mathrm{r}} \mathrm{G}=-\mathrm{nFE}, \Delta_{\mathrm{r}} \mathrm{G}^{0}=-\mathrm{nFE}_{\mathrm{cell}}^{0}$
$-\mathrm{nF} \Delta_{\mathrm{r}} \mathrm{E}_{\text {cell }}=-\mathrm{nF} \Delta_{\mathrm{r}} \mathrm{E}_{\text {cell }}^{0}+\mathrm{RT} \ln \mathrm{Q}$
$\mathrm{E}_{\text {cell }}=\mathrm{E}_{\text {cell }}^{0}-\frac{0.0591}{\mathrm{n}} \log \frac{1}{\left[\mathrm{Br}^{-}\right]^{2}\left[\mathrm{H}^{+}\right]^{2}}$
$=(0-1.08)-\frac{0.0591}{2} \log \frac{1}{(0.010)^{2}(0.030)^{2}}$
$=-1.08-0.02955 \times \log \frac{1}{9 \times 10^{-8}}$
$\therefore \mathrm{E}_{\mathrm{cell}}=0-(1.08)-\frac{0.0591}{2} \log \left(1.111 \times 10^{7}\right)$
$=-1.08-\frac{0.0592}{2}(7.0457)$
$=-1.09-0.208$
$\therefore \mathrm{E}_{\text {cell }}=-1.288 \mathrm{~V}$
Question 6. In the button cells widely used in watches and other devices the following reaction takes place:
$\mathrm{Zn}(\mathrm{s})+\mathrm{Ag}_{2} \mathrm{O}(\mathrm{s})+\mathrm{H}_{2} \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{Zn}^{2+}(\mathrm{aq})+2 \mathrm{Ag}(\mathrm{s})+2 \mathrm{OH}^{-}(\mathrm{aq})$
Determine $\Delta_{\mathrm{r}} \mathrm{G}^{0}$ and $\mathrm{E}^{0}$ for the reaction. [ $\left.2 \mathrm{Marks}\right]$
Solution. At anode: $\mathrm{Zn}_{(\mathrm{s})} \rightarrow \mathrm{Zn}_{(\mathrm{aq})}^{2+}+2 \mathrm{e}^{-} ; \mathrm{E}^{0}=0.76 \mathrm{~V}$
At anode: $A g_{2} O(s)+H_{2} O(1)+2 e^{-} \rightarrow 2 A g(s)+20 H^{-}(a q) ; E^{0}=0.344 V$
Overall reaction:
$\mathrm{Zn}(\mathrm{s})+\mathrm{Ag}_{2} \mathrm{O}(\mathrm{s})+\mathrm{H}_{2} \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{Zn}^{2+}(\mathrm{aq})$
$+2 \mathrm{Ag}(\mathrm{s})+2 \mathrm{OH}^{-}(\mathrm{aq}) ; \mathrm{E}^{0}=1.104 \mathrm{~V}$
$E_{\text {cell }}^{0}=E_{\text {cathode }}^{0}-E_{\text {anode }}^{0}$
Formula:
$\Delta_{\mathrm{r}} \mathrm{G}^{0}=-\mathrm{nFE}^{0}$
$=-2 \times 96500 \times 1.04$
$=-213043.296 \mathrm{~J}$
$=-213.04 \mathrm{k} \mathrm{J}$
Question 7. Define conductivity and molar conductivity for the solution of an electrolyte. Discuss their variation with concentration. [3 Marks]
Solution. Conductivity of a solution is defined as the conductance of a solution of $1 \mathrm{~cm}$ in length and area of cross-section $1 \mathrm{~cm} 2$ The inverse of resistivity is called
conductivity or specific conductance. It is represented by the symbol $\mathrm{K}$. If $\rho$ is resistivity, then we can write:
$\mathrm{K}=\frac{1}{\rho}$
The conductivity of a solution at any given concentration is the conductance (G) of one unit volume of solution kept between two platinum electrodes with the unit area of cross-section and at a distance of unit length.
i.e., $\mathrm{G}=\mathrm{K} \frac{\mathrm{a}}{1}=\mathrm{K}$
$\mathrm{G}=\mathrm{K} \quad$ (since $\mathrm{a}=1, \mathrm{l}=1)$
Conductivity weak and strong electrolytes always decreases with a decrease in concentration, This is because the number of ions per unit volume that carry the current in a solution decreases as decrease in concentration of electrolyte.
Molar conductivity:
Molar conductivity of a solution at a given concentration is the conductance of volume (V) of a solution containing 1 mole of the electrolyte kept between two electrodes with the area of cross-section A and distance of unit length.
$\Lambda_{\mathrm{m}}=\mathrm{K} \frac{\mathrm{A}}{\mathrm{l}}$
Now, $1 \mathrm{~A}=\mathrm{V}$ (volume containing $1 \mathrm{~mole}$ of the electrolyte).
$\therefore \Lambda_{\mathrm{m}}=\mathrm{KV}$
Molar conductivity increases with a decrease in concentration. This is because the total volume $\mathrm{V}$ of the solution containing one mole of the electrolyte increases on dilution.
The variation of $\Lambda_{\mathrm{m}}$ with $\sqrt{\mathrm{c}}$ for strong and weak electrolytes is shown in the following plot:
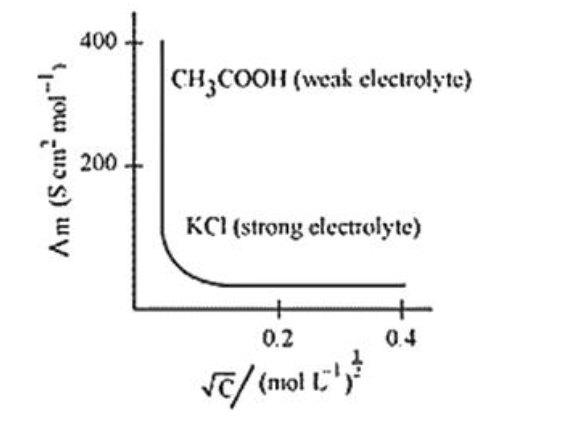
Question 8. The conductivity of $0.20 \mathrm{M}$ solution of $\mathrm{KCl}$ at $298 \mathrm{~K}$ is $0.0248 \mathrm{Scm}^{-1}$. Calculate its molar conductivity. [2 Marks]
Solution: Given,
$\mathrm{K}=0.0248 \mathrm{~S} \mathrm{~cm}^{-1}$
$\mathrm{c}=0.20 \mathrm{M}$
Formula use: Molar conductivity $\left(\Lambda_{\mathrm{m}}\right)=\frac{\mathrm{K} \times 1000}{\mathrm{c}}$
$\Lambda_{\mathrm{m}}=\frac{0.0248 \times 1000}{0.2}$
$\Lambda_{\mathrm{m}}=124 \mathrm{Scm}^{2} \mathrm{~mol}^{-1}$
Concept insight:
Important from examination point.
Question 9. The resistance of a conductivity cell containing $0.001 \mathrm{M} \mathrm{KCl}$ solution at $298 \mathrm{~K}$ is $1500 \Omega$. What is the cell constant if conductivity of $0.001 \mathrm{M} \mathrm{KCl}$ solution at $298 \mathrm{~K}$ is $0.146 \times 10^{-3} \mathrm{~S} \mathrm{~cm}^{-1} .[2 \mathrm{Marks}]$
Solution. Given,
Conductivity $(\mathrm{K})=0.146 \times 10^{-3} \mathrm{~S} \mathrm{~cm}^{-1}$
Resistance $(\mathrm{R})=1500 \Omega$
$\therefore$ Cell constant $=\mathrm{K} \times \mathrm{R}$
$=0.146 \times 10^{-3} \times 1500$
$=0.219 \mathrm{~cm}^{-1}$
Concept insight:
$\mathrm{K}=\frac{\text { cell constant }}{\mathrm{R}}$
Question 10. The conductivity of sodium chloride at $298 \mathrm{~K}$ has been determined at different concentrations and the results are given below:
Concentration/M $0.0010 .0100 .020 \quad 0.0500 .100$
$10^{2} \times \mathrm{k} / \mathrm{S} \mathrm{m}^{-1} 1.237 \quad 11.8523 .1555 .53106 .74$
Calculate $\Lambda_{\mathrm{m}}$ for all concentrations and draw a plot between $\Lambda_{\mathrm{m}}$ and $\mathrm{c} \frac{1}{2} .$ Find the value of $\Lambda_{\mathrm{m}}^{0} .[4$ Marks $]$
Solution. Given,
$\mathrm{K}=1.237 \times 10^{-2} \mathrm{~S} \mathrm{~m}^{-1}, \mathrm{c}=0.001 \mathrm{M}$
Then $\mathrm{c}^{\frac{1}{2}}=(0.0316)^{\frac{1}{2}}$
$\therefore \Lambda_{\mathrm{m}}=\frac{\mathrm{K}}{\mathrm{c}}$
$=\frac{1.237 \times 10^{-4} \mathrm{~S} \mathrm{~cm}^{-1}}{0.001 \mathrm{~mol} \mathrm{~L}^{-1}} \times \frac{1000 \mathrm{~cm}^{3}}{\mathrm{~L}}$
$=123.7 \mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}$
Given,
$\mathrm{K}=11.85 \times 10^{-2} \mathrm{~S} \mathrm{~m}^{-1}, \mathrm{c}=0.010 \mathrm{M}$
Then, $\mathrm{K}=11.85 \times 10^{-4} \mathrm{~S} \mathrm{~cm}^{-1}, \mathrm{c}^{\frac{1}{2}}=(0.1)^{\frac{1}{2}}$
$\therefore \Lambda_{\mathrm{m}}=\frac{\mathrm{K}}{\mathrm{c}}$
$=\frac{11.85 \times 10^{-4} \mathrm{~S} \mathrm{~cm}^{-1}}{0.010 \mathrm{~mol} \mathrm{~L}^{-1}} \times \frac{1000 \mathrm{~cm}^{3}}{\mathrm{~L}}$
$=118.5 \mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}$
Given,
$\mathrm{K}=23.15 \times 10^{-2} \mathrm{Sm}^{-1}, \mathrm{c}=0.020 \mathrm{M}$
Then, $\mathrm{K}=23.15 \times 10^{-4} \mathrm{~S} \mathrm{~cm}^{-1}, \mathrm{c}^{\frac{1}{2}}=0.1414 \mathrm{M}^{\frac{1}{2}}$
$\therefore \Lambda_{\mathrm{m}}=\frac{\mathrm{K}}{\mathrm{c}}$
$=\frac{23.15 \times 10^{-4} \mathrm{~S} \mathrm{~cm}^{-1}}{0.020 \mathrm{~mol} \mathrm{~L}^{-1}} \times \frac{1000 \mathrm{~cm}^{3}}{\mathrm{~L}}$
$=115.8 \mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}$
Given,
$\mathrm{K}=55.53 \times 10^{-2} \mathrm{~S} \mathrm{~m}^{-1}, \mathrm{c}=0.050 \mathrm{M}$
Question 11. Conductivity of $0.00241 \mathrm{M}$ acetic acid is $7.896 \times 10^{-5} \mathrm{~S} \mathrm{~cm}^{-1} .$ Calculate its molar conductivity and if $\Lambda_{\mathrm{m}}^{0}$ for acetic acid is $390.5 \mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}$, what is its dissociation constant? [3 Marks]
Solution: Given,
$\mathrm{K}=7.896 \times 10^{-5} \mathrm{~S} \mathrm{~m}^{-1}$
$\mathrm{c}=0.00241 \mathrm{~mol} \mathrm{~L}^{-1}=\frac{0.00241}{1000} \mathrm{~mol} \mathrm{~cm}^{-3}$
Then, molar conductivity, $\Lambda_{\mathrm{m}}=\frac{\mathrm{K}}{\mathrm{c}}$
$=\frac{7.896 \times 10^{-5} \mathrm{~S} \mathrm{~cm}^{-1}}{0.00241 \mathrm{~mol} \mathrm{~L}^{-1}} \times \frac{1000 \mathrm{~cm}^{3}}{\mathrm{~L}}$
$=32.76 \mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}$
Again, $\Lambda_{\mathrm{m}}^{0}$ for acetic acid $=390.5 \mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}$
Now, degree of dissociation $(\alpha)=\frac{\Lambda_{\mathrm{m}}}{\Lambda_{\mathrm{m}}}^{0}$
$\alpha=\frac{\Lambda_{\mathrm{m}}}{\Lambda_{\mathrm{m}}^{0}}=\frac{32.76 \mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}}{390.5 \mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}}$
$=0.084$
Let assume HA is weak acid and it undergoes dissociate
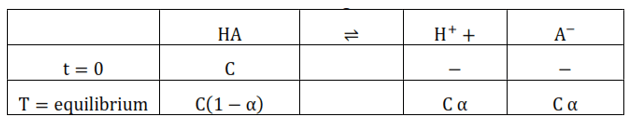
$\therefore$ Dissociation constant for acid $\left(\mathrm{K}_{\mathrm{a}}\right)$
$=\frac{\left(\mathrm{H}^{+}\right)\left(\mathrm{A}^{-}\right)}{(\mathrm{HA})}=\frac{\mathrm{C} \alpha \times \mathrm{C} \alpha}{\mathrm{C}(1-\alpha)}=\frac{\mathrm{c} \alpha^{2}}{(1-\alpha)}$
$=\frac{\left(0.00241 \mathrm{~mol} \mathrm{~L}^{-1}\right)(0.084)^{2}}{(1-0.084)}$
$=1.86 \times 10^{-5} \mathrm{~mol} \mathrm{~L}^{-1}$
Question 12. How much charge is required for the following reductions?
(i) $1 \mathrm{~mol}$ of $\mathrm{Al}^{3+}$ to $\mathrm{Al}$
(ii) $1 \mathrm{~mol}$ of $\mathrm{Cu}^{2+}$ to $\mathrm{Cu}$
(iii) $1 \mathrm{~mol}$ of $\mathrm{MnO}_{4}^{-}$to $\mathrm{Mn}^{2+}$ [3 Marks]
Solution: (i) $1 \mathrm{~mol}$ of $\mathrm{Al}^{3+}$ to Al required 3 mole of electron $=3$
$\mathrm{Al}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Al}$
$\therefore$ Required charge $=3 \mathrm{~F}$
$=3 \times 96500 \mathrm{C}$
$=289500 \mathrm{C}$
(ii) $\mathrm{Cu}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cu}$
$1 \mathrm{~mol}$ of $\mathrm{Cu}^{2+}$ to Cu required 2 mole of electron
$\therefore$ Required charge $=2 \mathrm{~F}$
$=2 \times 96500 \mathrm{C}$
$=193000 \mathrm{C}$
(iii) $\mathrm{MnO}_{4}^{-} \rightarrow \mathrm{Mn}^{2+}$
1 mol of $\mathrm{MnO}_{4}^{-}$to $\mathrm{Mn}^{2+}$ required 5 mole of electron
i.e., $\mathrm{Mn}^{7+}+5 \mathrm{e}^{-} \rightarrow \mathrm{Mn}^{2+}$
$\therefore$ Required charge $=5 \mathrm{~F}$
$=5 \times 96500 \mathrm{C}$
$=482500 \mathrm{C}$
Concept insight:
Write the balanced equation and then calculate the charge.
Question 13. How much electricity in terms of Faraday is required to produce?
(i) $20.0 \mathrm{~g}$ of $\mathrm{Ca}$ from molten $\mathrm{CaCl}_{2}$
(ii) $40.0 \mathrm{~g}$ of $\mathrm{Al}$ from molten $\mathrm{Al}_{2} \mathrm{O}_{3}$ [2 Marks]
Solution: (i) for $20.0 \mathrm{~g}$ of Ca from molten
$\mathrm{CaCl}_{2} 1 \mathrm{~mol}$ of $\mathrm{CaCl}_{2}$ to Ca required 5 mole of electron
$\mathrm{Ca}^{2+}+2 \mathrm{e}^{-1} \rightarrow \mathrm{Ca}_{40 \mathrm{~g}}$
Electricity required to produce $40 \mathrm{~g}$ of calcium $=2 \mathrm{~F}$
Therefore, electricity required to produce $20 \mathrm{~g}$ of calcium $=\frac{2 \times 20}{40} \mathrm{~F}$
$=1 \mathrm{~F}=96500 \mathrm{C}$
(ii) $40.0 \mathrm{~g}$ of Al from molten $\mathrm{Al}_{2} \mathrm{O}_{3}$
1 mole of Al from molten $\mathrm{Al}_{2} \mathrm{O}_{3} \quad$ required 3 mole of electron
$\mathrm{Al}^{3+}+3 \mathrm{e}^{-} \rightarrow \underset{27 \mathrm{~g}}{\mathrm{Al}}$
Electricity required to produce $27 \mathrm{~g}$ of $\mathrm{Al}=3 \mathrm{~F}$
Therefore, electricity required to produce $40 \mathrm{~g}$ of $\mathrm{Al}=\frac{3 \times 40}{27} \mathrm{~F}=4.44$
$=4.44 \mathrm{~F}=4.44 \times 96500=428460 \mathrm{C}$
Question 14. How much electricity is required in coulomb for the oxidation of
(i) $1 \mathrm{~mol}$ of $\mathrm{H}_{2} \mathrm{O}$ to $\mathrm{O}_{2}$
(ii) 1 mol of $\mathrm{FeO}$ to $\mathrm{Fe}_{2} \mathrm{O}_{3}$ [2 Marks]
Solution: (i) $1 \mathrm{~mol}$ of $\mathrm{H}_{2} \mathrm{O}$ to $\mathrm{O}_{2}$
$\mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{H}_{2}+\frac{1}{2} \mathrm{O}_{2}$
Now, we can write:
$0^{2-} \rightarrow \frac{1}{2} \mathrm{O}_{2}+2 \mathrm{e}^{-}$
$1 \mathrm{~mol}$ of $\mathrm{H}_{2} \mathrm{O}$ to $\mathrm{O}_{2}$ required $2 \mathrm{~mole}$ of electron
Electricity required for the oxidation of $1 \mathrm{~mol}$ of $\mathrm{H}_{2} \mathrm{O}$ to $\mathrm{O}_{2}=2 \mathrm{~F}$
$=2 \times 96500 \mathrm{C}$
$=193000 \mathrm{C}$
(ii) $1 \mathrm{~mol}$ of $\mathrm{FeO}$ to $\mathrm{Fe}_{2} \mathrm{O}_{3}$
$\mathrm{Fe}^{2+} \rightarrow \mathrm{Fe}^{3+}+\mathrm{e}^{-1}$
$1 \mathrm{~mol}$ of $\mathrm{Fe} 0$ to $\mathrm{Fe}_{2} \mathrm{O}_{3}$ required 1 mole of electron
Electricity required for the oxidation of $1 \mathrm{~mol}$ of $\mathrm{FeO}$ to $\mathrm{Fe}_{2} \mathrm{O}_{3}=1 \mathrm{~F}$
$=96500 \mathrm{C}$
Question 15. A solution of $\mathrm{Ni}\left(\mathrm{NO}_{3}\right)_{2}$ is electrolyzed between platinum electrodes using a current of 5 amperes for 20 minutes. What mass of $\mathrm{Ni}$ is deposited at the cathode? [2 Marks]
Solution. Given,
Current $=5 \mathrm{~A}$
Time $=20 \times 60=1200 \mathrm{~s}$
formula : $\operatorname{Charge}(\mathrm{Q})=\operatorname{current}(\mathrm{i}) \times \operatorname{time}(\mathrm{t})$ in second
$=5 \times 1200$
$=6000 \mathrm{C}$
According to the question
$\mathrm{Ni}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Ni}(\mathrm{s})$
$\mathrm{Ni}^{2+}$ to Ni required 2 mole of electron
Current required $=2 \mathrm{~F}$
Nickel deposited by $2 \times 96500 \mathrm{C}=58.71 \mathrm{~g}$
Therefore, nickel deposited by $6000 \mathrm{C}=\frac{58.71 \times 6000}{2 \times 96500} \mathrm{~g}$
$=1.825 \mathrm{~g}$
Hence, $1.825 \mathrm{~g}$ of nickel will be deposited at the cathode.
Question 16. Three electrolytic cells A, B, C containing solutions of $\mathrm{ZnSO}_{4}, \mathrm{AgNO}_{3}$ and $\mathrm{CuSO}_{4}$, respectively are connected in series. A steady current of $1.5$ amperes was passed through them until $1.45 \mathrm{~g}$ of silver deposited at the cathode of cell $\mathrm{B}$. How long did the current flow? What mass of copper and zinc were deposited? [3 Marks]
Solution. For electrolyte 1 :
$\mathrm{Zn}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \underset{65.4 \mathrm{~g}}{\mathrm{Zn}(\mathrm{s})}$
i.e., $2 \times 96500 \mathrm{C}$ of charge deposit $=65.4 \mathrm{~g}$ of $\mathrm{Zn}$
Therefore, $1295.43 \mathrm{C}$ of charge will deposit $=\frac{65.4 \times 1295.43}{2 \times 96500} \mathrm{~g}$
$=0.439 \mathrm{~g}$ of $\mathrm{Zn}$
For electrolyte $2: \mathrm{Ag}^{+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{Ag}(\mathrm{s})$
i.e., 1 mole of $\mathrm{Ag}$ is deposited by $1 \mathrm{~F}$ charge
i.e., $108 \mathrm{~g}$ of $\mathrm{Ag}$ is deposited by $96500 \mathrm{C}$.
Therefore, $1.45 \mathrm{~g}$ of $\mathrm{Ag}$ is deposited by $=\frac{96500 \times 1.45}{108} \mathrm{C}$
$=1295.6 \mathrm{C}$
Given,
Current $=1.5 \mathrm{~A}$
$\therefore$ Time $=\frac{1295.43}{1.5} \mathrm{~s}$
$=863.6 \mathrm{~s}$
$=864 \mathrm{~s}=\frac{864}{60} \min =14.40 \mathrm{~min}$
For electrolyte $3:$
$\mathrm{Cu}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \underset{63.5 \mathrm{~g}}{\mathrm{Cu}(\mathrm{s})}$
1 mole of $\mathrm{Cu}$ is deposited by $2 \mathrm{~F}$ charge
i.e., $2 \times 96500 \mathrm{C}$ of charge deposit $=63.5 \mathrm{~g}$ of $\mathrm{Cu}$
Therefore, $1295.43 \mathrm{C}$ charge will deposit $=\frac{63.5 \times 1295.43}{2 \times 96500} \mathrm{~g}$
$=0.426 \mathrm{~g}$ of $\mathrm{Cu}$
\mathrm{Zn}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \underset{65.4 \mathrm{~g}}{\mathrm{Zn}(\mathrm{s})}
1 mole of $\mathrm{Zn}$ is deposited by $2 \mathrm{~F}$ charge
i.e., $2 \times 96500 \mathrm{C}$ of charge deposit $=65.4 \mathrm{~g}$ of $\mathrm{Zn}$
Therefore, $1295.43 \mathrm{C}$ of charge will deposit $=\frac{65.4 \times 1295.43}{2 \times 96500} \mathrm{~g}$
$=0.4389 \mathrm{~g}$ of $\mathrm{Zn}$
Question 17. Using the standard electrode potentials given in table predict if the reaction between the following is feasible: $[5$ Marks $]$
(i) $\mathrm{Fe}^{3+}(\mathrm{aq})$ and $\mathrm{I}^{-}(\mathrm{aq})$
(ii) $\mathrm{Ag}^{+}(\mathrm{aq})$ and $\mathrm{Cu}(\mathrm{s})$
(iii) $\mathrm{Fe}^{3+}(\mathrm{aq})$ and $\mathrm{Br}^{-}(\mathrm{aq})$
(iv) $\mathrm{Ag}(\mathrm{s})$ and $\mathrm{Fe}^{3+}(\mathrm{aq})$
(v) $\mathrm{Br}_{2}$ (aq) and $\mathrm{Fe}^{2+}(\mathrm{aq})$
Solution. (i) $\left.\quad \mathrm{Fe}^{3+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{Fe}^{2+}(\mathrm{aq})\right] \mathrm{E}^{0}=+0.77 \mathrm{~V} \quad$ [equation 1]
$\begin{array}{lll}2 \mathrm{I}^{-}(\mathrm{aq}) \rightarrow \mathrm{I}_{2}(\mathrm{~s})+2 \mathrm{e}^{-} & \mathrm{E}^{0}=-0.54 \mathrm{~V} & \text { [equation 2] }\end{array}$
[equation 1] $\times 2+$ [equation 2]
Final reaction $2 \mathrm{Fe}^{3+}(\mathrm{aq})+2 \mathrm{I}^{-}(\mathrm{aq}) \rightarrow 2 \mathrm{Fe}^{2+}(\mathrm{aq})+\mathrm{I}_{2}(\mathrm{~s}) ; \quad \mathrm{E}^{0}=+0.23 \mathrm{~V}$
Since $E^{0}$ for the overall reaction is positive, the reaction between $\mathrm{Fe}^{3+}(\mathrm{aq})$ and $\mathrm{I}^{-}(\mathrm{aq})$ is feasible.
(ii) $\left.\quad \mathrm{Ag}^{+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{Ag}(\mathrm{s})\right] \mathrm{E}^{0}=+0.80 \mathrm{~V} \quad$ [equation 1]
$\mathrm{Cu}(\mathrm{s}) \rightarrow \mathrm{Cu}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \quad \mathrm{E}^{0}=-0.34 \mathrm{~V} \quad$ [equation
[equation 1] $\times 2+$ [equation 2]
Final reaction $2 \mathrm{Ag}^{+}(\mathrm{aq})+\mathrm{Cu}(\mathrm{s}) \rightarrow 2 \mathrm{Ag}(\mathrm{s})+\mathrm{Cu}^{2+}(\mathrm{aq}) ; \quad \mathrm{E}^{0}=+0.46 \mathrm{~V}$
Since $E^{0}$ for the overall reaction is positive, the reaction between $A g_{(a q)}^{+}$and $\mathrm{Cu}_{(\mathrm{s})}$ is feasible.
(iii) $\left.\mathrm{Fe}^{3+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{Fe}^{2+}(\mathrm{aq})\right] \mathrm{E}^{0}=+0.77 \mathrm{~V}$ [equation 1$]$
$2 \mathrm{Br}^{-}(\mathrm{aq}) \rightarrow \mathrm{Br}_{2}(\mathrm{l})+2 \mathrm{e}^{-} \mathrm{E}^{0}=-1.09 \mathrm{~V} \quad$ [equation2]
[equation 1] $\times 2+[$ equation 2]
$2 \mathrm{Fe}^{3+}(\mathrm{aq})+2 \mathrm{Br}^{-}(\mathrm{aq}) \rightarrow 2 \mathrm{Fe}^{2+}(\mathrm{aq})$
$+\mathrm{Br}_{2}(\mathrm{l}) ; \quad \mathrm{E}^{0}=-0.32 \mathrm{~V}$
Since $E^{0}$ for the overall reaction is negative, the reaction between $\mathrm{Fe}^{3+}(\mathrm{aq})$ and $\mathrm{Br}^{-}(\mathrm{aq})$ is not feasible.
(iv) $\mathrm{Ag}(\mathrm{s}) \rightarrow \mathrm{Ag}^{+}(\mathrm{aq})+\mathrm{e}^{-} ; \mathrm{E}^{0}=-0.80 \mathrm{~V} \quad$ [equation 1]
$\mathrm{Fe}^{3+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{Fe}^{2+}(\mathrm{aq}) ; \mathrm{E}^{0}=+0.77 \mathrm{~V}[$ equation 2$]$
Add equation 1 and equation 2
Final reaction
$\mathrm{Ag}(\mathrm{s})+\mathrm{Fe}^{3+}(\mathrm{aq}) \rightarrow \mathrm{Ag}^{+}(\mathrm{aq})+\mathrm{Fe}^{2+}(\mathrm{aq}) ; \quad \mathrm{E}^{0}=-0.03 \mathrm{~V}$
Since $E^{0}$ for the overall reaction is negative, the reaction between $\mathrm{Ag}(\mathrm{s})$ and $\mathrm{Fe}^{3+}(\mathrm{aq})$ is not feasible.
(v) $\mathrm{Br}_{2}$ (aq) $+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Br}^{-}(\mathrm{aq}) ; \mathrm{E}^{0}=+1.09 \mathrm{~V} \quad$ [equation 1]
$\left.\mathrm{Fe}^{2+}(\mathrm{aq}) \rightarrow \mathrm{Fe}^{3+}(\mathrm{aq})+\mathrm{e}^{-}\right] ; \mathrm{E}^{0}=-0.77 \mathrm{~V} \quad$ [equation 2]
[equation 1] $+[$ equation 2] $\times 2$
Final reaction :
$\mathrm{Br}_{2}(\mathrm{aq})+2 \mathrm{Fe}^{2+}(\mathrm{aq}) \rightarrow 2 \mathrm{Br}^{-}(\mathrm{aq})$
$+2 \mathrm{Fe}^{3+}(\mathrm{aq}) ; \mathrm{E}^{0}=+0.32 \mathrm{~V}$
Question 18. Predict the products of electrolysis in each of the following:
(i) An aqueous solution of $\mathrm{AgNO}_{3}$ with silver electrodes
(ii) An aqueous solution of $\mathrm{AgNO}_{3}$ with platinum electrodes
(iii) A dilute solution of $\mathrm{H}_{2} \mathrm{SO}_{4}$ with platinum electrodes
(iv) An aqueous solution of $\mathrm{CuCl}_{2}$ with platinum electrodes. [4 Marks]
Solution: (i) At cathode:
The following reduction reactions compete to take place at the cathode.
$\mathrm{Ag}^{+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{Ag}(\mathrm{s}) ; \mathrm{E}^{0}=0.80 \mathrm{~V}$
$\mathrm{H}^{+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \frac{1}{2} \mathrm{H}_{2}(\mathrm{~g}) ; \mathrm{E}^{0}=0.00 \mathrm{~V}$
The reaction with a higher value of $E^{0}$ takes place at the cathode. Therefore, deposition of silver will take place at the cathode.
At anode:
Since $\mathrm{Pt}$ electrodes are inert, the anode is not attacked by $\mathrm{N} \mathrm{O}_{3}^{-}$ions. Therefore, $\mathrm{OH}^{-}$or $\mathrm{NO}_{3}^{-}$ions can be oxidized at the anode. But $\mathrm{OH}^{-}$ions having a lower discharge potential and get preference and decompose to liberate $O_{2}$
$\mathrm{OH}^{-} \rightarrow \mathrm{OH}+\mathrm{e}^{-}$
$4 \mathrm{OH}^{-} \rightarrow 2 \mathrm{H}_{2} \mathrm{O}+\mathrm{O}_{2}$
(iii) At the cathode, the following reduction reaction occurs to produce $\mathrm{H}_{2}$ gas,
$\mathrm{H}^{+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \frac{1}{2} \mathrm{H}_{2}(\mathrm{~g})$
At the anode, the following processes are possible.
$2 \mathrm{H}_{2} \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{O}_{2}(\mathrm{~g})+4 \mathrm{H}^{+}(\mathrm{aq})+4 \mathrm{e}^{-} \mathrm{E}^{\circ}=+1.23 \mathrm{~V}$
$2 \mathrm{SO}_{4}^{2-}(\mathrm{aq}) \rightarrow \mathrm{S}_{2} \mathrm{O}_{6}^{2}-(\mathrm{aq})+2 \mathrm{e}^{-} \quad \mathrm{E}^{0}=+1.96 \mathrm{~V}$
The formation of oxidation is preferred because the discharge potential of oxygen is lower than that of $\mathrm{S}_{2} \mathrm{O}_{6}^{2-}$.
The following reactions are possible at the cathode.
$\mathrm{Cu}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Cu}(\mathrm{s}) \mathrm{E}^{\mathrm{o}}=0.43 \mathrm{~V}$
$\mathrm{H}^{+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \frac{1}{2} \mathrm{H}_{2}(\mathrm{~g}) \mathrm{E}^{\mathrm{o}}=0.00 \mathrm{~V}$
At
Also Read,
Class 12 Chemistry Notes Free PDF Download.
Class 12 Chemistry Book Chapterwise Free PDF Download.
Class 12 Chemistry Exemplar Chapterwise Free PDF Download.
If you have any Confusion related to NCERT Solutions for Class 12 Chemistry Chapter 3 Electrochemistry PDF then feel free to ask in the comments section down below.
To watch Free Learning Videos on Class 12 Chemistry by Kota’s top IITan’s Faculties Install the eSaral App
