JEE Main Previous Year Papers Questions of Chemistry With Solutions are available at eSaral.
Simulator
Previous Years AIEEE/JEE Mains Questions
Q. The set representing the correct order of ionic radius is ?
(1) $\mathrm{Li}^{+}>\mathrm{Na}^{+}>\mathrm{Mg}^{2+}>\mathrm{Be}^{2+}$
(2) $\mathrm{Mg}^{2+}>\mathrm{Be}^{2+}>\mathrm{Li}^{+}>\mathrm{Na}^{+}$
(3) $\mathrm{Li}^{+}>\mathrm{Be}^{2+}>\mathrm{Na}^{+}>\mathrm{Mg}^{2+}$
(4) $\mathrm{Na}^{+}>\mathrm{Li}^{+}>\mathrm{Mg}^{2+}>\mathrm{Be}^{2+}$
[AIEEE 2009]
Ans. (4)
Q. The correct order of electron gain enthalpy with negative sign of F, Cl, Br and I, having atomic number 9, 17, 35 and 53 respectively, is ?
(1) I > Br > Cl > F
(2) F > Cl > Br > I
(3) Cl > F > Br > I
(4) Br > Cl > I > F
[AIEEE 2011]
Ans. (3)
Q. The increasing order of the ionic radii of the given isoelectronic species is ?
(1) $\mathrm{K}^{+}, \mathrm{S}^{2-}, \mathrm{Ca}^{2+}, \mathrm{Cl}^{-}$
(2) $\mathrm{Cl}^{-}, \mathrm{Ca}^{2+}, \mathrm{K}^{+}, \mathrm{S}^{2-}$
(3) $\mathrm{S}^{2-}, \mathrm{Cl}^{-}, \mathrm{Ca}^{2+}, \mathrm{K}^{+}$
(4) $\mathrm{Ca}^{2+}, \mathrm{K}^{+}, \mathrm{Cl}^{-}, \mathrm{S}^{2-}$
[AIEEE 2012]
Ans. (4)
Q. Which of the following represents the correct order of increasing first ionization enthalpy for Ca, Ba, S, Se and Ar ?
(1) Ca < S < Ba < Se < Ar
(2) S < Se < Ca < Ba < Ar
(3) Ba < Ca < Se < S < Ar
(4) Ca < Ba S < Se < Ar
[JEE-Main 2013]
Ans. (3)
Q. The first ionisation potential of Na is 5.1 eV. The value of electron gain enthalpy of $\mathrm{Na}^{+}$ will be?
(1) – 2.55 eV
(2) – 5.1 eV
(3) – 10.2 eV
(4) + 2.55 eV
[JEE-Main 2013]
Ans. (4)
Q. Electron gain enthalpy with negative sign of fluorine is less than that of chlorine due to:
(1) Smaller size of chlorine atom
(2) Bigger size of 2p orbital of fluorine
(3) High ionization enthalpy ol fluorine
(4) Smaller size of fluorine atom
[JEE-MAIN 2013 (OnLine)]
Ans. (4)
Q. The order of increasing sizes of atomic radii among the elements O, S, Se and As is ?
(1) As < S < O < Se
(2) O < S < As < Se
(3) Se < S < As < O
(4) O < S < Se < As
[JEE-MAIN 2013 (OnLine)]
Ans. (4)
Q. Which is the correct order of second ionization potential of C, N, O and F in the following?
(1) O > F > N > C
(2) O > N > F > C
(3) C > N > O > F
(4) F > O > N > C
[JEE-MAIN 2013 (OnLine)]
Ans. (1)
Q. Which of the following series correctly represents relations between the elements from X to Y ?
 [JEE-MAIN 2014 (OnLine)]
[JEE-MAIN 2014 (OnLine)]
 [JEE-MAIN 2014 (OnLine)]
[JEE-MAIN 2014 (OnLine)]
Ans. (3)
Q. Which of the following atoms has the highest first ionization energy ?
(1) Sc
(2) Rb
(3) Na
(4) K
[JEE-MAIN 2016]
Ans. (1)
Due to poor shielding of d-electrons in Sc, $Z_{\mathrm{eff}}$ of Sc becomes more so that ionisation energy of Sc is more than Na, K and Rb.
Q. The group having isoelectronic species is :-
(1) $\mathrm{O}^{2-}, \mathrm{F}^{-}, \mathrm{Na}^{+}, \mathrm{Mg}^{2+}$
(2) $\mathrm{O}^{-}, \mathrm{F}^{-}, \mathrm{Na}, \mathrm{Mg}^{+}$
(3) $\mathrm{O}^{2-}, \mathrm{F}^{-}, \mathrm{Na}, \mathrm{Mg}^{2+}$
(4) $\mathrm{O}^{-}, \mathrm{F}^{-}, \mathrm{Na}^{+}, \mathrm{Mg}^{2+}$
[JEE-MAIN 2017]
Ans. (1)
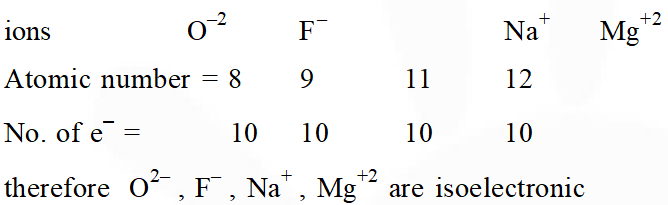
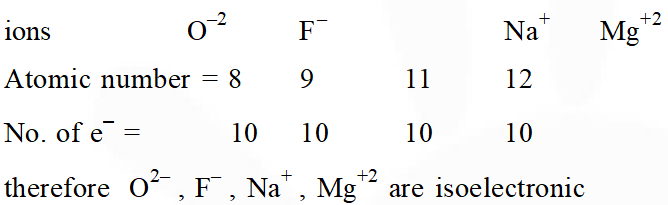
Comments
international pharmacy
Oct. 28, 2023, 6:35 a.m.
This website certainly has all of the information and facts I needed concerning this subject and didn't know who to ask.
PRANJAL
March 11, 2023, 7:29 p.m.
your 5th question's answer is wrong . its answer is -5.1 , because the electron is added in na+ . so the value will we just reversed and it will be -5.1 .
I think you guys should check the answer and then post , this would lead in a bad way to students . not expected from you guys . E saral should look after this .
THANK YOU
PRANJAL
Priyanka
Dec. 5, 2022, 11:29 a.m.
Can you develop more facilities in this link like choosing correct answer and find marks with negative marking like exam
Dec. 5, 2022, 11:28 a.m.
Can you develop more facilities in this link like choosing correct answer and find marks with negative marking like exam
koiovbi
April 16, 2021, 11:13 a.m.
EUNRUNIM69LCQ]Y WE8 8JNXD]
27 IU PU
5'PH,67H
HK68
6
HKGW
KKLG
E5K
Nikita Sharma
Dec. 13, 2020, 6:50 p.m.
Good questions....but more high level based questions should be uploaded for iit jee advanced level preparation.
Sachin Gupta
Sept. 6, 2020, 11:50 a.m.
Awesome I strong my concepts I would correct all questions ok thanks a lot.
Naga Sri harsha kuncharapu
Aug. 29, 2020, 12:40 p.m.
Thank you
I became strong in concept
Upload more questions please
Aviral Saxena
Aug. 23, 2020, 2:15 a.m.
If i scored full then how is my base strong in this topic. Also update the ques and bring ques of diff concepts
Abha soni
June 4, 2020, 8:06 p.m.
But still really helpful thankyou so much for your efforts for us in free.😊
