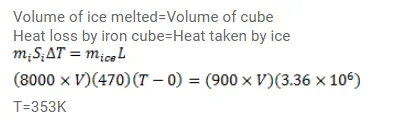Question:
A cube of iron (density $=8000 \mathrm{~kg} / \mathrm{m}^{3}$, specific heat capacity $=470 \mathrm{~J} / \mathrm{kg}-\mathrm{K}$ ) is heated to a high temperature and is placed on a large block of ice at $0^{\circ} \mathrm{C}$. The cube melts the ice below it, displaces the water and sinks. In the final equilibrium position, its upper surface just goes inside the ice. Calculate the initial temperature of the cube. Neglect any loss of heat outside the ice and the cube. The density of ice=9000 $\mathrm{kg} / \mathrm{m}^{3}$ and the latent heat of fusion of ice $=3.36 \times 10^{5} \mathrm{~J} / \mathrm{kg}$.
Solution: