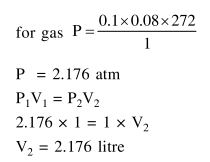Question:
A cylinder containing an ideal gas $(0.1$ mol of $1.0 \mathrm{dm}^{3}$ ) is in thermal equilibrium with a large volume of $0.5$ molal aqueous solution of ethylene glycol at its freezing point. If the stoppers $S_{1}$ and $S_{2}$ (as shown in the figure) are suddenly withdrawn, the volume of the gas in litres after equilibrium is achieved will be
(Given, $\mathrm{K}_{\mathrm{f}}$ (water) $=2.0 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}{ }^{-1}$,
$\mathrm{R}=0.08 \mathrm{dm}^{3}$ atm $\left.\mathrm{K}^{-1} \mathrm{~mol}^{-1}\right)$
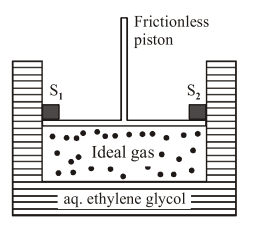
Solution: