Question:
A graph of vapour pressure and temperature for three different liquids $X, Y$, and $Z$ is shown below:
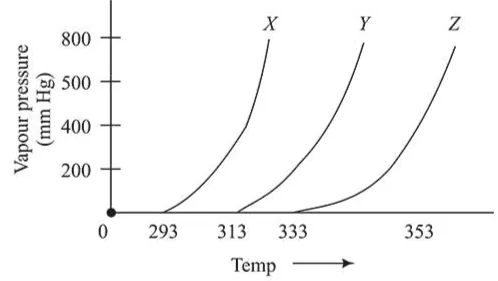
The following inferences are made:
(A) $X$ has higher intermolecular interactions compared to $\mathrm{Y}$.
(B) $X$ has lower intermolecular interactions compared to $\mathrm{Y}$.
(C) $Z$ has lower intermolecular interactions compared to Y.
The correct inference(s) is/are:
Correct Option: , 3
Solution:
At a particular temperature as intermolecular force of attraction increases vapour pressure decreases.
Thus, intermolecular forces are inversely proportional to vapour pressure and directly proportional to temperature.
Therefore X has lower intermolecular interactions compared to Y.
