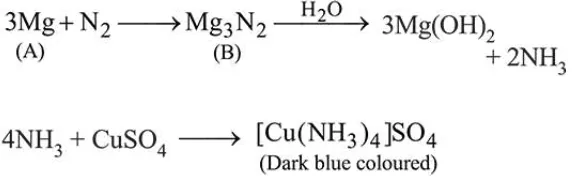
Question:
A metal (A) on heating in nitrogen gas gives compound B.
B on treatment with $\mathrm{H}_{2} \mathrm{O}$ gives a colourless gas which when passed through $\mathrm{CuSO}_{4}$ solution gives a dark blueviolet coloured solution. A and B respectively, are:
Correct Option: , 3
Solution: