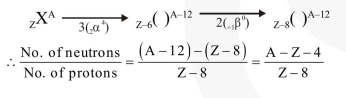Question:
A radioactive nucleus (initial mass number $A$ and atomic number $Z$ ) emits $3 \alpha$-particles and 2 positrons. The ratio of number of neutrons to that of protons in the final nucleus will be:-
Correct Option: , 3
Solution: