Question:
A solution of phenol in chloroform when treated with aqueous $\mathrm{NaOH}$ gives compound $\mathrm{P}$ as a major product. The mass percentage of carbon in $\mathrm{P}$ is________. (to the nearest integer)
(Atomic mass : $\mathrm{C}=12 ; \mathrm{H}=1 ; \mathrm{O}=16$ )
Solution:
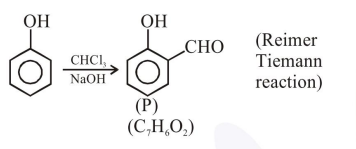
Molecular weight of $\mathrm{C}_{7} \mathrm{H}_{6} \mathrm{O}_{2}=122$
$\% \mathrm{C}=\frac{12 \times 7 \times 100}{122}=68.85 \approx 69$
