Question:
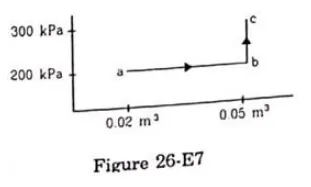
A substance is taken through the process abc as shown in figure (26-E7). If the internal energy of the substance increases by $5000 \mathrm{~J}$ and a heat of $2625 \mathrm{cal}$ is given to the system, calculate the value of J.
Solution:

