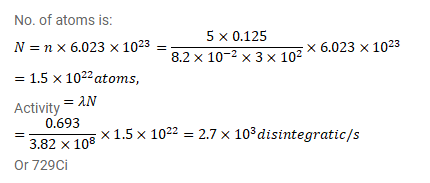Question:
A vessel of volume $125 \mathrm{~cm}^{3}$ contains tritium $\left({ }^{3} \mathrm{H}, \mathrm{t}_{1 / 2}=12.3 \mathrm{y}\right)$ at $500 \mathrm{kPa}$ and $300 \mathrm{k}$. Calculate the activity of the gas. PV=nRT= $\frac{m}{n} R T$
PV=NKT
Solution: