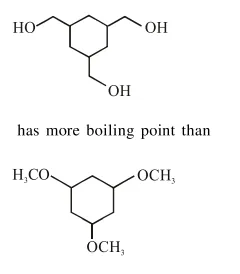
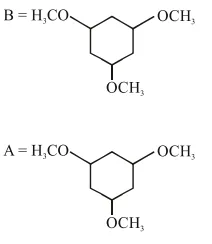
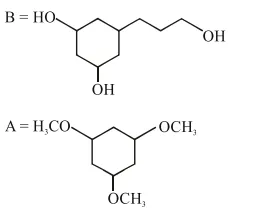
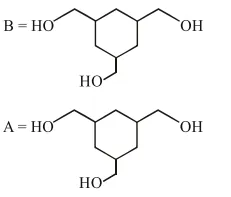
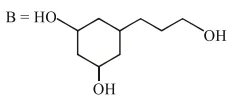
Question:
Among the compounds $A$ and $B$ with molecular formula $\mathrm{C}_{9} \mathrm{H}_{18} \mathrm{O}_{3}, \mathrm{~A}$ is having higher boiling point the B. The possible structures of $A$ and $B$ are :
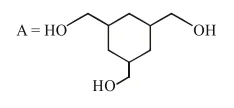
Correct Option: 1
Solution:
Alcohol has more boiling point than ether (due to hydrogen bonding).
$\mathrm{So}$,