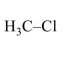Question:
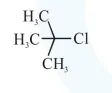
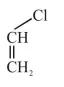
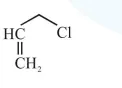
Among the following compounds, which one has the shortest $\mathrm{C}-\mathrm{Cl}$ bond ?
Correct Option: , 3
Solution:
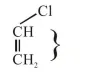
In option (3) $\mathrm{C}-\mathrm{Cl}$ bond is shortest due to resonance of lone pair of $-\mathrm{Cl}$.
Due to resonance $\mathrm{C}-\mathrm{Cl}$ bond acquire partial double bond character.
Hence $\mathrm{C}-\mathrm{Cl}$ bond length is least.