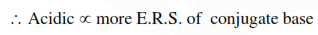Question: Among the following oxoacids, the correct decreasing order of acid strength is :
$\mathrm{HClO}_{4}>\mathrm{HClO}_{3}>\mathrm{HClO}_{2}>\mathrm{HOCl}$
$\mathrm{HClO}_{2}>\mathrm{HClO}_{4}>\mathrm{HClO}_{3}>\mathrm{HOCl}$
$\mathrm{HOCl}>\mathrm{HClO}_{2}>\mathrm{HClO}_{3}>\mathrm{HClO}_{4}$
$\mathrm{HClO}_{4}>\mathrm{HOCl}>\mathrm{HClO}_{2}>\mathrm{HClO}_{3}$
Correct Option: 1
Solution: