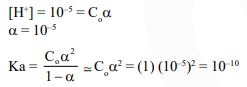Question: An acid HA ionises as
$\mathrm{HA} \rightleftharpoons \mathrm{H}^{+}+\mathrm{A}^{-}$
The $\mathrm{pH}$ of $1.0 \mathrm{M}$ solution is 5 . Its dissociation constant would be :-
$1 \times 10^{-10}$
5
$5 \times 10^{-8}$
$1 \times 10^{-5}$
Correct Option: 1
Solution: