Question:
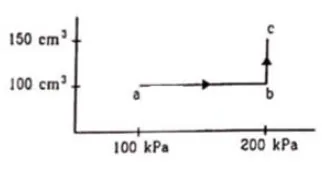
An ideal gas $(\gamma=1.67)$ is taken through the process abc shown in fig. The temperature at the point a is $300 \mathrm{~K}$. Calculate
(a) the temperature at $b$ and $c,
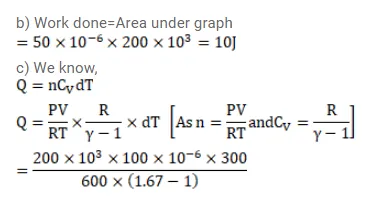
(b)$ the work done in the process,
(c) the amount of heat supplied in the path $a b$ and in the path bc and
(d) the change in the internal energy of the gas in the process.
Solution: