Question:
Arrange the following compounds in increasing order of $\mathrm{C}$ - OH bond length: methanol, phenol, $p$-ethoxyphenol
Correct Option:
Solution:
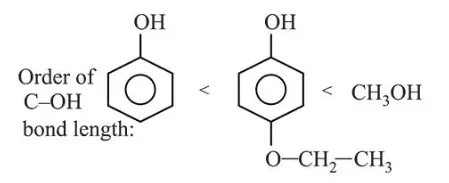
Resonance is a deciding factor to determine the order of bond length in given compounds. Phenol exhibits least $\mathrm{C}-\mathrm{OH}$ bond length due to resonance whereas methanol will show maximum bond length due to lack of resonance and $p$-ethoxyphenol will have some intermediate value of bond length.
