At 300 K and 1 atm, 15 mL of a gaseous hydrocarbon requires 375 mL air containing 20% O2 by volume for complete combustion.
Question:
At 300 K and 1 atm, 15 mL of a gaseous hydrocarbon requires 375 mL air containing 20% O2 by volume for complete combustion. After combustion the gases occupy 330 mL. Assuming that the water formed is in liquid form and the volumes were measured at the same temperature and pressure, the formula of the hydrocarbon is :-
Correct Option:
Solution:
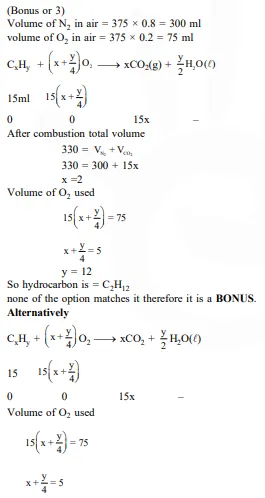
If further information (i.e., 330 ml) is neglected, option (3) only satisfy the above equation.
Comments
GaRBH LaBaT
May 4, 2023, 4:29 p.m.
you cannot say say that only 30ml of CO2 is evolved....because some amount of it will dissolve in water. Instead i did the solution as:
Initial volume= final volume
15+375=330 + V(water)
=>V(water)=60 ml
Now, 15y/2=60
y=8
putting it in the equation derived above
x=3 and hence C3H8 is the hydrocarbon
GaRBH LaBaT
May 4, 2023, 6:53 a.m.
you cannot say say that only 30ml of CO2 is evolved....because some amount of it will dissolve in water. Instead i did the solution as:
Initial volume= final volume
15+375=330 + V(water)
=>V(water)=60 ml
Now, 15y/2=60
y=8
putting it in the equation derived above
x=3 and hence C3H8 is the hydrocarbon
