Question:
Statement I : Sodium hydride can be used as an oxidising agent.
Statement II : The lone pair of electrons on nitrogen in pyridine makes it basic.
Choose the CORRECT answer from the options given below:
Correct Option: , 3
Solution:
(1) $\mathrm{NaH}$ (sodium Hydride) is used as a reducing reagent.
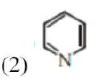
In pyridine, due to free electron on $\mathrm{N}$ atom, it is basic in nature.
Hence statement $\mathrm{I}$ is false $\backslash$ II is true.
