Question:
Consider the following plots of rate constant versus $\frac{1}{T}$ for four different reactions. Which of the following orders is correct for the activation energies of these reactions?
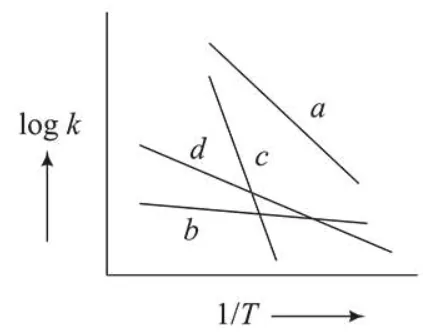
Correct Option: , 3
Solution:
Arrhenius equation, $k=\mathrm{A} e^{-\mathrm{Ea} / \mathrm{RT}}$
$\log k=\log \mathrm{A}-\frac{\mathrm{E}_{a}}{2.303 \mathrm{RT}}$
slope $=-\frac{E_{a}}{2.303 R}$
$\therefore$ More negative the slope greater will be the $E_{a}$
So correct order is $\mathrm{E}_{c}>\mathrm{E}_{a}>\mathrm{E}_{d}>\mathrm{E}_{b}$
