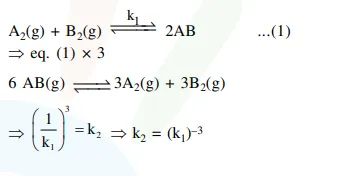Question:
Consider the following reversible chemical reactions :
$\mathrm{A}_{2}(\mathrm{~g})+\mathrm{Br}_{2}(\mathrm{~g}) \stackrel{\mathrm{k}_{1}}{\rightleftharpoons} 2 \mathrm{AB}(\mathrm{g}) \ldots . .(1)$
$6 \mathrm{AB}(\mathrm{g}) \stackrel{\mathrm{K}_{2}}{\rightleftharpoons} 3 \mathrm{~A}_{2}(\mathrm{~g})+3 \mathrm{~B}_{2}(\mathrm{~g}) \ldots . .(2)$
The relation between $K_{1}$ and $K_{2}$ is :
Correct Option: , 2
Solution: