Question:
Consider the given plots for a reaction obeying Arrhenius equation $\left(0^{\circ} \mathrm{C}<\mathrm{T}<300^{\circ} \mathrm{C}\right)$ : $(\mathrm{k}$ and $\mathrm{E}_{\mathrm{a}}$ are rate constant and activation energy, respectively)
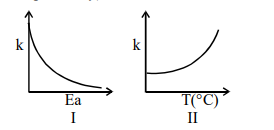
Choose the correct option :
Correct Option: , 4
Solution:
On increasing $E_{a}, K$ decreases
