Consider the reaction:
$\mathrm{Cl}_{2}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{~S}(\mathrm{aq}) \rightarrow \mathrm{S}(\mathrm{s})+2 \mathrm{H}^{+}(\mathrm{aq})+2 \mathrm{Cl}^{-}(\mathrm{aq})$
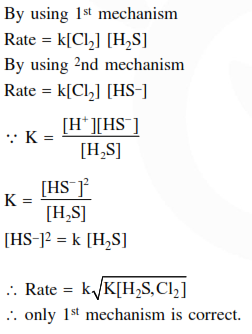
The rate equation for this reaction is rate $=\mathrm{k}\left[\mathrm{Cl}_{2}\right]\left[\mathrm{H}_{2} \mathrm{~S}\right]$
Which of these mechanisms is/are consistent with this rate equation?
1. $\mathrm{Cl}_{2}+\mathrm{H}_{2} \mathrm{~S} \rightarrow \mathrm{H}^{+}+\mathrm{Cl}^{-}+\mathrm{Cl}^{+}+\mathrm{HS}^{-}$(slow)
$\mathrm{Cl}^{+}+\mathrm{HS}^{-} \rightarrow \mathrm{H}^{+}+\mathrm{Cl}^{-}+\mathrm{S}($ fast $)$.
2. $\mathrm{H}_{2} \mathrm{~S} \Leftrightarrow \mathrm{H}^{+}+\mathrm{HS}^{-}$(fast equilibrium)
$\mathrm{Cl}_{2}+\mathrm{HS}^{-} \rightarrow 2 \mathrm{Cl}^{-}+\mathrm{H}^{+}+\mathrm{S}($ slow $)$
Correct Option: 1