Question: Consider the reaction sequence given below :
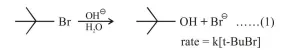
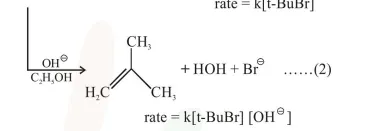
Which of the following statements is true :
Changing the concentration of base will have no effect on reaction (1)
Changing the concentration of base will have no effect on reaction (2)
Changing the base from $\mathrm{OH}^{\ominus}$ to $^{\ominus} \mathrm{OR}$ will have no effect on reaction (2)
Doubling the concentration of base will double the rate of both the reactions.
Correct Option: 1
Solution:
Reaction 1: SN,
Reaction $2: \mathrm{E}_{2}$
$\mathrm{SN}_{1}$ is independent of concentration of nucleophile/base