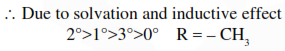Question: Considering the basic strength of amines in aqueous solution, which one has the smallest $\mathrm{pK}_{\mathrm{b}}$ value ?
$\left(\mathrm{CH}_{3}\right)_{3} \mathrm{~N}$
$\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}$
$\left(\mathrm{CH}_{3}\right)_{2} \mathrm{NH}$
$\mathrm{CH}_{3} \mathrm{NH}_{2}$
Correct Option: , 3
Solution: