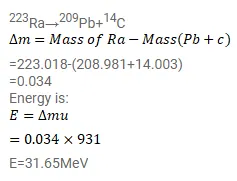Question:
Find the energy liberated are as follows:
223Ra→209Pb+14C
The atomic masses needed are as follows:
$\begin{array}{lll}{ }^{223} \mathrm{Ra} & { }^{209} \mathrm{~Pb} & { }^{14} \mathrm{C} \\ 223.018 \mathrm{u} & 208.981 \mathrm{u} & 14.003 \mathrm{u}\end{array}$
Solution: