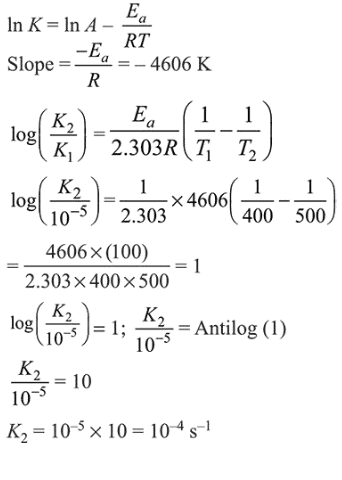Question:
For a reaction, consider the plot of $\ln \mathrm{k}$ versus $1 / \mathrm{T}$ given in the figure. If the rate constant of this reaction at $400 \mathrm{~K}$ is $10^{-5} \mathrm{~s}^{-1}$, then the rate constant at $500 \mathrm{~K}$ is :
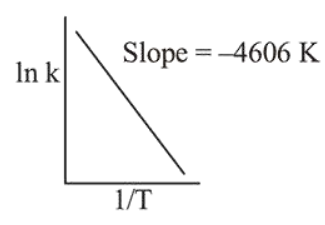
Correct Option: , 3
Solution:
From Arrhenius equation,