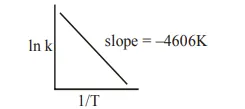
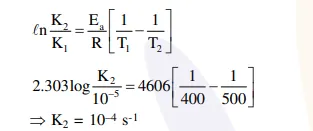Question: For a reaction consider the plot of $\ln \mathrm{k}$ versus 1/T given in the figure. If the rate constant of this reaction at $400 \mathrm{~K}$ is $10^{-5} \mathrm{~s}^{-1}$, then the rate constant at $500 \mathrm{~K}$ is :
$2 \times 10^{-4} \mathrm{~s}^{-1}$
$10-4 s^{-1}$
$10^{-6} \mathrm{~s}^{-1}$
$4 \times 10^{-4} \mathrm{~s}^{-1}$
Correct Option: , 2
Solution: