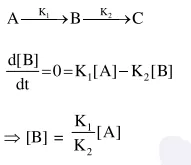
Question: For a reaction scheme $\mathrm{A} \stackrel{\mathrm{k}_{1}}{\longrightarrow} \mathrm{B} \stackrel{\mathrm{k}_{2}}{\longrightarrow} \mathrm{C}$, if
the rate of formation of B is set to be zero then the concentration of $B$ is given by :
$\left(\frac{\mathrm{k}_{1}}{\mathrm{k}_{2}}\right)[\mathrm{A}]$
$\left(\mathrm{k}_{1}+\mathrm{k}_{2}\right)[\mathrm{A}]$
$\mathrm{k}_{1} \mathrm{k}_{2}[\mathrm{~A}]$
$\left(\mathrm{k}_{1}-\mathrm{k}_{2}\right)[\mathrm{A}]$
Correct Option: 1
Solution: