Question:
For an electrochemical cell
$\mathrm{Sn}(\mathrm{s})\left|\mathrm{Sn}^{2+}(\mathrm{aq}, 1 \mathrm{M}) \| \mathrm{Pb}^{2+}(\mathrm{aq}, 1 \mathrm{M})\right| \mathrm{Pb}(\mathrm{s})$
the ratio $\frac{\left[\mathrm{Sn}^{2+}\right]}{\left[\mathrm{Pb}^{2+}\right]}$ when this cell attains
equilibrium is___________.
(Given $\mathrm{E}_{\mathrm{Sn}^{2} \mid \mathrm{|sn}}^{0}=-0.14 \mathrm{~V}$,
$\left.\mathrm{E}_{\mathrm{Pb}^{2+} \mid \mathrm{Pb}}^{0}=-0.13 \mathrm{~V}, \frac{2.303 \mathrm{RT}}{\mathrm{F}}=0.06\right)$
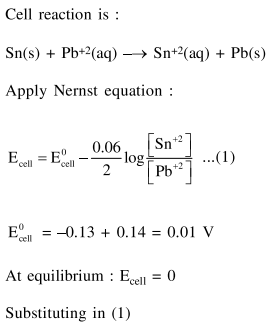
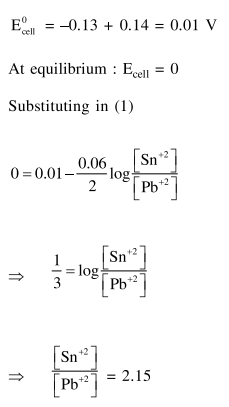
Solution: