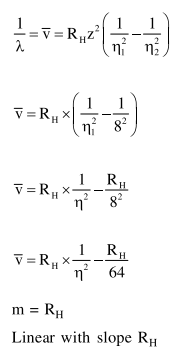Question:
For emission line of atomic hydrogen from $\mathrm{n}_{\mathrm{i}}=8$ to $\mathrm{n}_{\mathrm{f}}=$ the plot of wave number
$(\bar{v})$ against $\left(\frac{1}{n^{2}}\right)$ will be (The Ry dberg constant, $\mathrm{R}_{\mathrm{H}}$ is in wave number unit).
Correct Option: , 4
Solution: