Question: For the non-stoichiometre reaction $2 \mathrm{~A}+\mathrm{B} \rightarrow \mathrm{C}+\mathrm{D}$, the following kinetic data were obtained in three separate experiments, all at $298 \mathrm{~K}$.
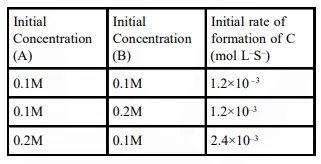
$\frac{\mathrm{dc}}{\mathrm{dt}}=\mathrm{k}[\mathrm{A}][\mathrm{B}]^{2}$
$\frac{\mathrm{dc}}{\mathrm{dt}}=\mathrm{k}[\mathrm{A}]$
$\frac{\mathrm{dc}}{\mathrm{dt}}=\mathrm{k}[\mathrm{A}][\mathrm{B}]$
$\frac{\mathrm{dc}}{\mathrm{dt}}=\mathrm{k}[\mathrm{A}]^{2}[\mathrm{~B}]$
Correct Option: , 2
Solution:
Solution not required