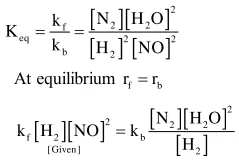Question:
For the reaction
$2 \mathrm{H}_{2}(\mathrm{~g})+2 \mathrm{NO}(\mathrm{g}) \rightarrow \mathrm{N}_{2}(\mathrm{~g})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{g})$
the observed rate expression is, rate $=\mathrm{k}_{\mathrm{f}}[\mathrm{NO}]^{2}\left[\mathrm{H}_{2}\right]$. The rate expression of the reverse reaction is :
Correct Option: , 4
Solution: