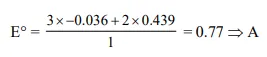Question:
Given :
$\mathrm{E}_{\mathrm{Fe}^{-3 /} / \mathrm{Fe}}^{\circ}=-0.036 \mathrm{~V}, \mathrm{E}_{\mathrm{Fe}^{\circ 2 /} / \mathrm{Fe}}^{\circ}=-0.439 \mathrm{~V}$. The value of standard electrode potential for the change.
$\mathrm{Fe}_{(\mathrm{aq})}^{+3}+\mathrm{e}^{-} \longrightarrow \mathrm{Fe}_{(\text {aq })}^{+2}$ will be :-
Correct Option:
Solution: