Question:
Given below are two statements:
Statement $\mathrm{I}:$ Both $\mathrm{CaCl}_{2} \cdot 6 \mathrm{H}_{2} \mathrm{O}$ and $\mathrm{MgCl}_{2} \cdot 8 \mathrm{H}_{2} \mathrm{O}$ undergo dehydration on heating.
Statement II : $\mathrm{BeO}$ is amphoteric whereas the oxides of other elements in the same group are acidic.
In the light of the above statements, choose the correct answer from the options given below :
Correct Option: , 2
Solution:
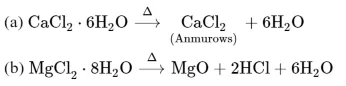
The dehydration of hydrated chloride of calcium can be achieved. The corresponding hydrated chloride of magnesium on heating suffer hydrolysis.

