Question:
________grams of 3-Hydroxy propanal $(\mathrm{MW}=74)$ must be dehydrated to produce $7.8 \mathrm{~g}$ of acrolein $(\mathrm{MW}=56)\left(\mathrm{C}_{3} \mathrm{H}_{4} \mathrm{O}\right)$ if the percentage yield is 64. (Round off to the Nearest Integer).
[Given : Atomic masses: C: $12.0 \mathrm{u}$, $\mathrm{H}: 1.0 \mathrm{u}, \mathrm{O}: 16.0 \mathrm{u}]$
Solution:
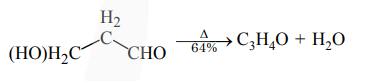
$\frac{\mathrm{x}}{74} \mathrm{~mol}$ $\frac{x}{74} \times 0.64=\frac{7.8}{56}$
$x=16.10$
$\simeq 16.00$
