Question:
Henry's constant (in kbar) for four gases $\alpha, \beta, \gamma$ and $\delta$ in water at $298 \mathrm{~K}$ is given below :
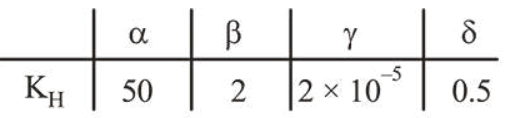
(density of water $=10^{3} \mathrm{~kg} \mathrm{~m}^{-3}$ at $298 \mathrm{~K}$ )
This table implies that :
Correct Option: , 4
Solution:
(a) From Henry's law $p=K_{\mathrm{H}}(x)$
Higher the value of $K_{\mathrm{H}}$ smaller will be the solubility of the gas, so $\gamma$ is more soluble.
(b) Though solubility of gases will decrease with increase in temperature but this conclusion can not be drawn from the given table.
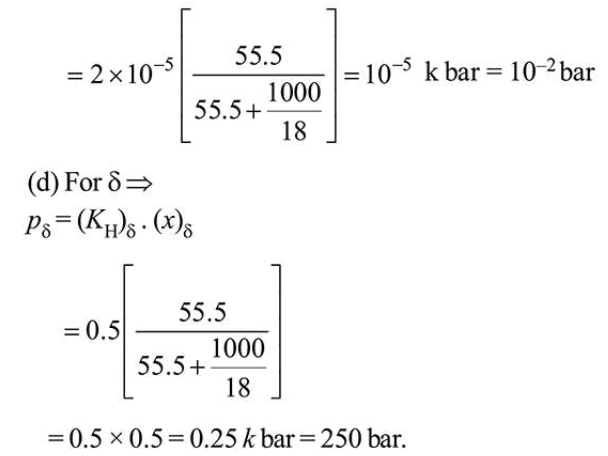
(c) For $\gamma$
$(p)_{\gamma}=\left(K_{\mathrm{H}}\right)_{\gamma} \cdot(x)_{\gamma}$