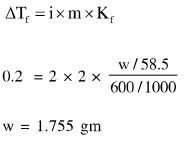Question:
How much amount of $\mathrm{NaCl}$ should be added to $600 \mathrm{~g}$ of water $(\rho=1.00 \mathrm{~g} / \mathrm{mL})$ to decrease the freezing point of water to $-0.2{ }^{\circ} \mathrm{C}$ ?
(The freezing point depression constant for water $=2 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1}$ )
Solution: